1. Background
Polycystic ovary syndrome (PCOS) occurs in 5% - 10% of all women during reproductive age and is distinguished by different symptoms of oligo/amenorrhea, androgen excess, insulin resistance, and typical morphology of polycystic ovarian (1). This syndrome is known as the most important reason for infertility via dysfunction of ovulatory. The fundamental etiology is not well understood but is reported to be multifactorial (1). The classical morphology of PCOS was described as hyperplasia, multiple cysts of follicular, thickening of ovarian cortical, and cessation of folliculogenesis, which is seen as multiple immature follicles (2).
The alteration of endocrine and local paracrine functions, in addition, changes in gene expression of cumulus and granulosa cells strongly impair the maturation competence of oocytes in patients with PCOS (3). Therefore, in vitro maturation (IVM) as an effective alternative method is suggested to improve the developmental competence of oocyte maturation (4, 5). The IVM of oocytes as a defined culture condition was used to promote the maturation of oocytes of women with PCOS, poor ovarian response to hormonal stimulation, and oncological problems (6).
Unfortunately, the suitable conditions for IVM of oocytes are still minimal due to the entire essential factors affecting IVM efficacy (7, 8). In this regard, α-Linolenic acid (ALA) belongs to the group of omega-3, which can affect oocyte’s growth and quality, and play an important role in the regulation of the meiotic stoppage at the stage of germinal vesicle (GV) (7).
Several studies have reported the positive effects of ALA on IVM media. For example, Marei et al. show cattle oocyte maturation, the quality, and development of cattle embryos were promoted in the IVM media treated with ALA (9). Also, the improvement of sheep blastocyst production and quality was observed in the presence of ALA (10).
A series of experiences must occur during folliculogenesis, which supports the future developmental competence of embryos. One of the most important ones in the mammalian oocytes is mitochondria. This organelle is amplified (~ 40 fold-increase) during the maturation phase (11). Therefore, the quality of oocytes is associated with mtDNA content (12). The study of Spikings et al. strongly confirmed that the developmental competence of oocytes, successful fertilization, and developmental progression are dependent on the correct expression of the mitochondrial transcription factor A (TFAM) gene (13). In another study, the expression of TFAM mRNA was significantly increased in mature oocytes compared to immature oocytes (14). Therefore, the evaluation of this gene in the oocytes matured in vitro in the presence of ALA antioxidant is necessary.
2. Objectives
With regards to the beneficial effects of ALA supplementation on developmental and maturation competence of the oocyte, this study was designed to clarify two questions, including (i) Whether treatment of IVM media with different dosages of ALA can promote oocyte IVM rate of mice with PCOS, and (ii) Whether the quality of PCOS oocytes matured in the presence of ALA improves?
3. Methods
3.1. Animals and Mouse Model with PCOS
Twenty NMRI female mice (30 - 35 g and 30 - 35 day-old) were purchased from Razi Institute, Iran. The animals were housed in a room with the controlled temperature at 22 ± 2ºC, relative humidity, and under a 12h light/12h dark cycle. Every four mice were housed in a cage with free access to food and water. The mice with PCOS injected with 4 mg/kg estradiol valerate (EV) dissolved in 0.2 mL sesame oil (Aburaihan Co., Iran) as an intramuscular (IM) for 60 consecutive days (15). Vaginal smears were obtained daily, and its epithelial changes were considered a sign of PCOS. In this way, the smears stained with Harris’ hematoxylin and eosin (H&E) were evaluated based on the vaginal cytology, and estrous, proestrus, metestrus, and diestrus stages studied. Regular cycles were defined as a duration of 4 - 5 days (16).
3.2. Examination of Ovarian Morphology
Sixty days after the EV injection, the mice were dislocated, and their ovaries removed. Then, the ovaries were fixed in 10% formalin, then embedded in paraffin, and cut serially in 5 μm sections. The mounted sections were also stained with H&E. The presence of the healthy (primary, preantral, antral, and preovulatory) and atretic follicles and corpus luteum (CL) were examined in the serial sections under a light microscope. Follicles are defined as primary (the presence of a single layer of cuboidal granulosa cells), preantral (1 - 2 small spaces filled by follicular fluid), antral (one large antral space), preovulatory (the presence of a rim of cumulus cells), and atretic (the presence of deformed follicle or the lack of oocyte or granulosa cells with pyknotic nuclei) (16).
3.3. Oocytes Collection and IVM
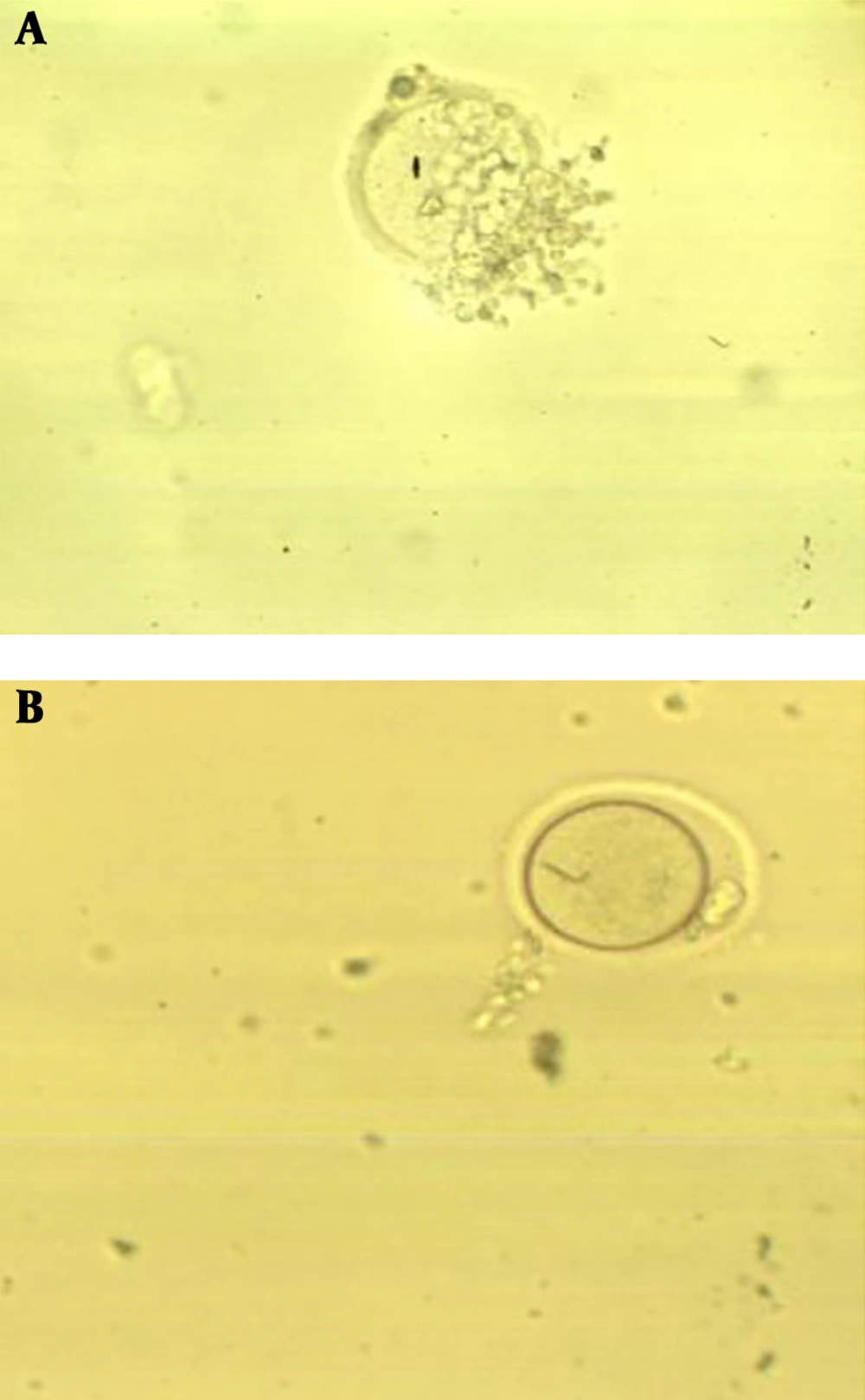
The ovaries were collected from mice with PCOS in preincubated alpha-modification of minimum essential medium (α-MEM). Then, the ovaries were dissected, and the germinal vesicle (GV) oocytes were cultured in α-MEM supplemented with 5% FCS and different concentrations of ALA (0 [control], 50, 100 µM) dissolved in Dimethyl Sulfoxide (DMSO) (17). Then, the oocytes were incubated at 37ºC in with 5% CO2. After 24 h of culture, the maturation of oocytes was assessed by an inverted microscope and was classified as GV, Metaphase I (MI), and metaphase II (MII).
3.4. Evaluation of Gene Expression
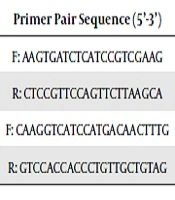
The MII oocytes were collected from the medium and kept at -196ºC until RNA extraction. Total RNA was extracted by RNX-plus solution, and then treatment was done with DNase, according to the manufacturer’s instructions. Using a cDNA synthesis kit, reverse transcription of total RNA of oocytes was done. Relative quantification was done in triplicate using quantitative real-time PCR and reactions using a mixture of SYBR1Green Supermix (Biofact) with cDNA equivalent to 1.5 oocytes and gene-specific primer (Table 1). The denaturation of template cDNA was performed at 95ºC for 10 min, then 35 cycles followed at 95ºC for 15 sec. The annealing temperature of gene-specific primer was carried out for 30 sec, and elongation was performed at 72ºC for 45 sec/60ºC for 30 min. Afterward, the melting curve analysis of samples was done to confirm the generation of a single specific product. Amplicon size was confirmed by safe stained-2% agarose gel electrophoresis. The expression of the GAPDH genes was considered endogenous reference, and oocytes from controls were used as calibrators. Calculations of relative quantification were performed with StepOne V2.1 software.
| Gene | Primer Pair Sequence (5’-3’) | Size (bp) |
|---|---|---|
| TFAM | F: AAGTGATCTCATCCGTCGAAG | 21 |
| R: CTCCGTTCCAGTTCTTAAGCA | 21 | |
| GAPDH | F: CAAGGTCATCCATGACAACTTTG | 23 |
| R: GTCCACCACCCTGTTGCTGTAG | 22 |
Primer Sequences Used in Quantitative Real-Time PCR
3.5. Statistical Analysis
Data are indicated as mean ± standard deviation or percent. Statistical analysis was performed using SPSS version 20 (IBM, Armonk, NY, USA). The tests of χ2 and One-way ANOVA was performed and followed by Tukey’s post-hoc tests to analyze differences among the groups and gene expression, respectively. The values of P < 0.05 were considered significant.
4. Results
4.1. Estrous Cycle and Ovary Morphology
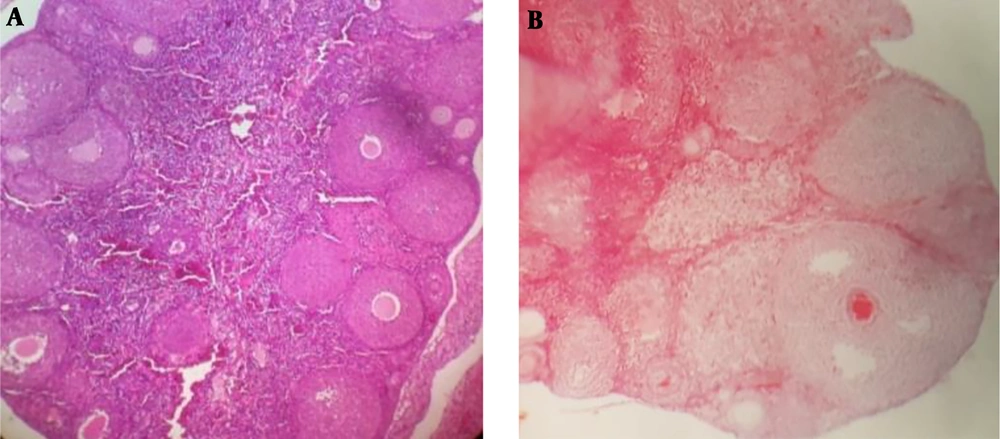
An essential feature of PCOS is the disruption of the estrous cycle. This feature was seen in the mice with PCOS (Figure 1). While the control mice showed a cycle of 4 - 5 days on a regular basis with the presence of CL. The vaginal smears of mice with PCOS had leukocytes (a symbol of constant pseudodiestrus and acyclic model). The histology of PCOS ovaries indicated atretic follicles without a sign of ovulation, absence of the CL and high early antral and antral follicles in comparison to the control group. The thickness of granulosa-theca cells in PCOS ovaries was significantly greater than the ones in the control group.
4.2. ALA Effects on IVM Rate of PCOS Oocytes
The maturation of oocytes was followed during the culture medium treated with different dosages of ALA (0, 50, and 100 µM). The results showed that the IVM rate of PCOS oocytes in the medium treated with 50 µM of ALA increased significantly in comparison to the control group (P = 0.009). The improved maturation rate can be attributed to oocyte quality. On the other hand, the higher dosage of ALA negatively affected the maturation of oocytes. Therefore, a dose-dependent pattern of ALA was established during the culture of PCOS oocytes (Figure 2 and Table 2).
4.3. Gene Expression Results
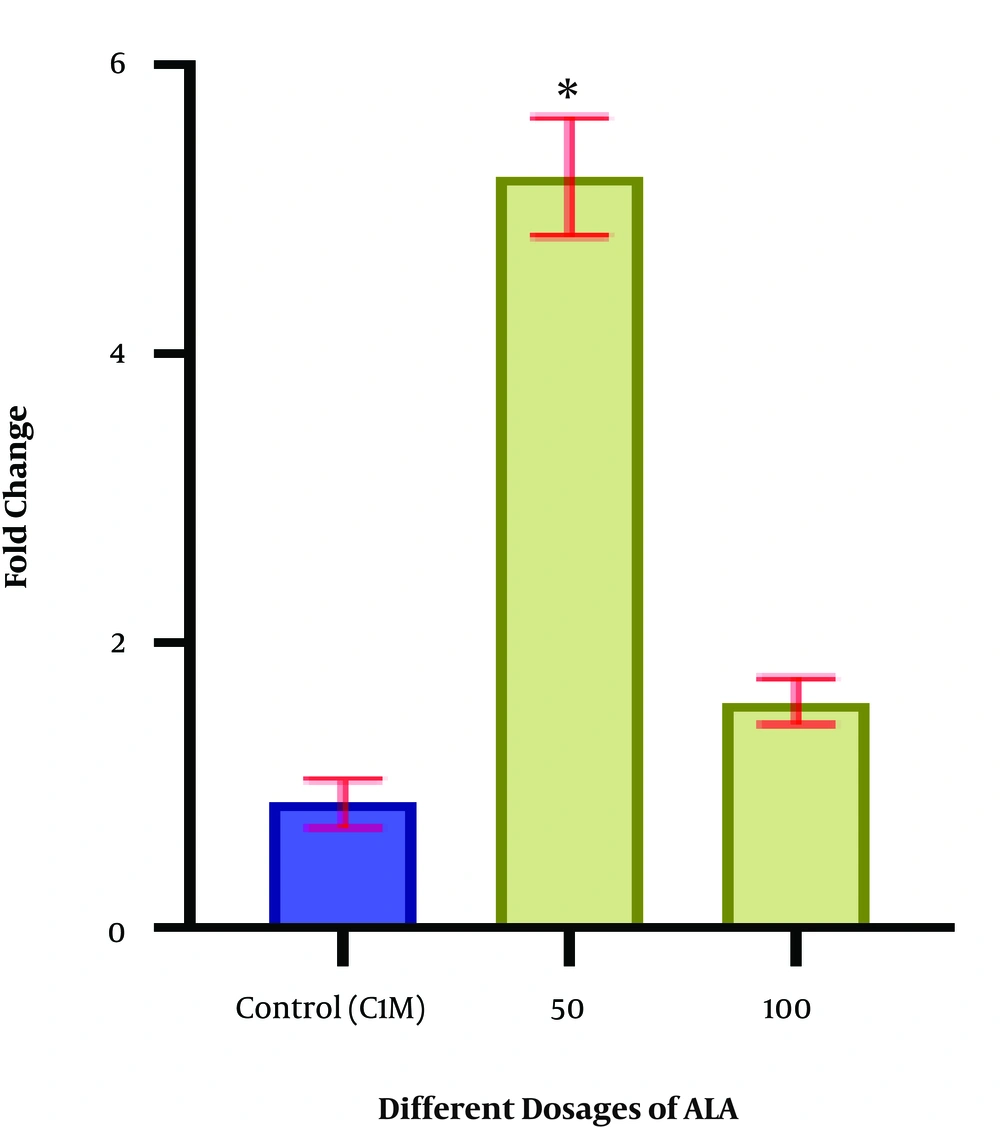
The ratio of expression of TFAM gene in the PCOS matured oocytes treated with 50 and 100 µM ALA, and the control group were 0.46 ± 0.09, 0.03 ± 0.09, and 0.025 ± 0.005, respectively (Figure 3). The rate of TFAM gene expression was significantly increased in the group treated with 50 µM of ALA (P < 0.05).
5. Discussion
In this study, an optimum range of ALA was detected to improve the in vitro maturation of PCOS oocytes. The results of this study showed that the presence of ALA not only improves the maturation rate of PCOS oocytes, but also promotes the mature oocyte quality so that the percentage of TFAM gene expression increases in the PCOS oocytes matured in the medium treated with ALA.
Different fatty acids have been detected in the follicular fluid of which the most majors are linoleic acid (LA), oleic acid (OA), stearic acid (SA), palmitic acid (PAL), and ALA (10). We tried to detect the effect of ALA on the in vitro oocyte maturation and to investigate its relationship with oocyte quality of mouse with PCOS.
In many studies, the IVM medium was treated with different dosages of ALA (e.g., 0, 10, 50, 100, or 200 µM) to evaluate its effect on cumulus cell expansion, the nuclear and cytoplasmic maturation of oocytes. These studies reported that supplementation of IVM medium with ALA at the highest concentration (100 µM) had a deleterious effect on cumulus cell expansion (9, 10, 17). The similar results of bovine oocyte maturation indicated by Marei et al. with a concentration of 50 µM of ALA (9). The study of Veshkini et al. reported that the maturation medium treated with 50 µM ALA improved the nuclear maturation of goat oocytes (10).
The critical factors in determining the developmental potential of fertilized oocytes are nuclear and cytoplasmic maturation. The diameter of follicles and oocytes are often small (18) and the vital factors in the cytoplasm of these oocytes are deficient (19). Preparation of the best maturation media could overcome some disadvantages and limitations of IVM conditions and promote their developmental potential (20). The previous studies indicated that follicular fluid contains large amounts of fatty acids. Therefore, each developmental phase of follicles needs fatty acids (21). Also, another study demonstrated that supplementation of IVM media with 50 µM ALA increased the maturation rate of goat oocytes (MII oocytes), while this dosage of ALA had no effect on oocyte’s cytoplasmic maturity (17). The study of Marei et al. found that maturation medium of bovine oocytes treated with 50 µM ALA increased the maturation rate; however, no effect was shown on cytoplasmic maturity (22). It seems that ALA increased both PGE2 and PGF2a levels in the culture media of oocytes. In fact, PGE2 has a major role in the nuclear maturation of oocytes and it plays as an important paracrine and/or autocrine regulator role in cumulus cells (23).
In this study, it was observed that the exogenous ALA in the IVM medium influenced on oocyte nuclear and cytoplasmic maturation. To the best of our knowledge, there are a few studies have ever evaluated the oocyte developmental competence in the presence of ALA (9, 10). However, such an effect has not been observed with PCOS oocytes of the mouse, and its role in oocyte cytoplasmic maturation is less clear until now. In this study, the ALA that was supplemented with the maturation medium affected oocyte gene expression patterns during IVM process. In addition, the treatment of oocyte with 50 µM ALA increased the frequency of TFAM gene compared with other groups showing that a direct association existed between treatment with the ALA and oocyte mitochondrial activity. Mitochondrial metabolic activity plays a critical role in the regulation of obvious signaling pathways during oocyte maturation (13). In addition, it has been reported an association between significant mtDNA replication and IVM of oocytes (transition from germinal vesicle to metaphase oocyte) (24). In this regard, TFAM activity was not only indicated to be critical for maintenance, replication and transcription of mtDNA, but also its transcriptional level was recently reported to positively associated with the oocyte maturation process (25, 26).
5.1. Conclusion
Overall, the results of the present study indicated that supplementation of maturation medium with 50 µM ALA increased IVM rate of PCOS oocytes. Also, ALA-treated medium led to a promotion in the quality of the PCOS oocyte via higher expression of TFAM gene. Therefore, the results of the study may improve the outcomes of assisted reproductive technique (ART) programs.