1. Background
Brain tumors arise from abnormal cellular growth within the brain and are classified as either benign or malignant. While benign tumors are non-cancerous and typically less aggressive, malignant brain tumors pose significant clinical challenges due to their invasive nature and proximity to critical brain structures. Globally, brain tumors represent the 19th most common cancer type, accounting for 1.9% of all cancers, and rank 12th in cancer-related mortality, contributing to 2.5% of cancer-related deaths, according to GLOBOCAN 2020 estimates (1). Radiation therapy remains a cornerstone in the management of brain tumors, with more than 50% of patients requiring it at some stages of treatment, according to reports of the World Health Organization (WHO) and the International Atomic Energy Agency (IAEA). However, delivering effective radiotherapy for brain tumors demands high precision to minimize damage to healthy tissues, particularly in critical regions (2, 3).
Radiation dose prescriptions for brain tumors vary depending on tumor type and location, ranging from 45 to 60 Gy. For example, benign tumors are treated with doses between 45 and 54 Gy, while malignant glioblastomas often require up to 60 Gy doses (4, 5). Whole-brain radiotherapy (WBRT) for metastases typically involves a total dose of 30 Gy delivered in 10 fractions (6). Dose constraints for sensitive structures, such as the brainstem, are generally limited to a maximum of 54 Gy to minimize the risk of complications (2, 7).
Advancements in radiotherapy techniques, such as intensity-modulated radiation therapy (IMRT) and volumetric-modulated arc therapy (VMAT), have significantly improved the radiation treatment precision and clinical outcomes. The IMRT utilizes advanced computer algorithms to generate customized radiation dose distributions, allowing for precise tumor targeting while minimizing exposure to adjacent healthy tissues. Tailored radiation dose distributions enable precise tumor targeting while sparing adjacent healthy tissues. Building upon IMRT, VMAT incorporates continuous gantry rotation to deliver modulated radiation beams, achieving highly conformal dose distributions, particularly beneficial for irregularly shaped tumors (4-6). These technologies are further enhanced by image-guided radiation therapy (IGRT), which integrates imaging into both treatment planning and delivery. This integration improves by accounting for patient positioning and anatomical changes in real time (7, 8).
New tomotherapy systems (Radixact®, Accuray, USA) offer a unique integration of IGRT and intensity modulation among modern radiotherapy modalities (3). These systems employ a helical delivery method using a ring gantry, enabling radiation to be delivered from multiple angles with sub-millimeter precision. Tomotherapy’s design enables the creation of highly conformal treatment plans, particularly for complex or irregular targets, and is effective in treating multiple lesions or extended fields, such as in total body irradiation. However, due to its fixed couch angle, the system is limited to delivering coplanar beams, restricting its ability to implement noncoplanar beam configurations. Yet, noncoplanar beam orientation is a critical feature for certain brain tumors, where oblique beam angles are necessary to achieve optimal dose conformity and organ sparing (8, 9).
Noncoplanar techniques, which deliver radiation beams from multiple angles outside a single plane, enhance dose distribution and improve dose conformity (10-12). Studies, such as the one by Yuasa and Kurosaki, have demonstrated the feasibility and dosimetric advantages of implementing noncoplanar approaches in tomotherapy using innovative head and neck fixtures (13). These studies highlight reduced radiation doses to organs at risk (OARs) and improved target coverage in phantom models.
2. Objectives
To address the inherent limitations of the tomotherapy system, this study introduces a novel, custom-designed baseplate capable of tilting the patient’s head in both yaw and pitch directions. This innovation aims at enhancing the system’s versatility in treating brain tumors by enabling noncoplanar beam arrangements, thereby improving dosimetric outcomes and sparing critical organs. This study evaluates the performance of the dedicated baseplate, paving the way for future advancements in tomotherapy applications.
3. Methods
3.1. Head Immobilization and CT Imaging
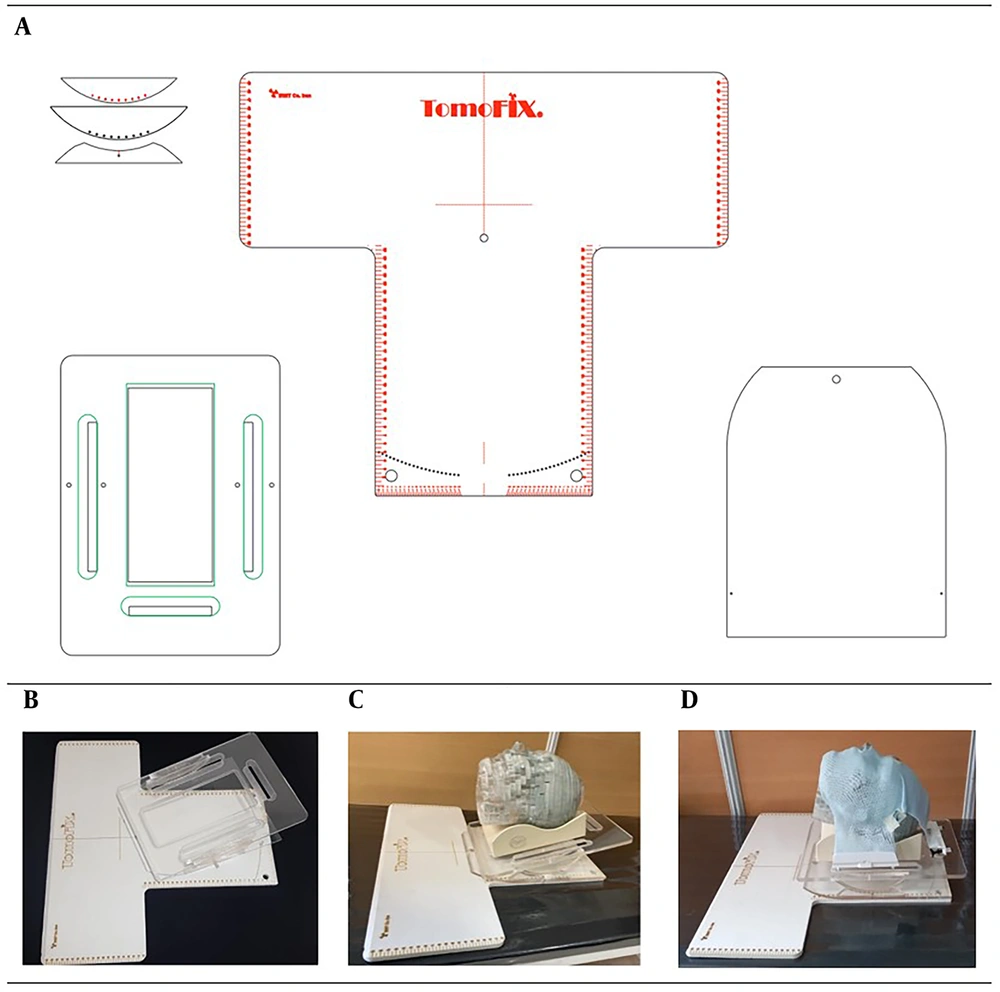
A new head base plate was developed to enable the use of noncoplanar fields in the treatment of brain tumors. The base plate is designed to allow controlled pitch and yaw movements, enhancing its versatility for complex radiotherapy setups. Constructed from durable materials, including Plexiglas and PVC, the base plate provides a stable and robust platform to ensure precise and reproducible patient positioning. The design of the base plate components and the constructed prototype are illustrated in Figure 1A and 1B.
As illustrated in Figure 1A-D , the base plate allows adjustable pitch movements from -20° to +20° in 5° increments and yaw movements from -42° to +42° in 2° increments. This fine adjustability is crucial for achieving optimal beam angles in cases that require noncoplanar field arrangements to improve dose distribution conformity, such as hypophysis malignancies or stereotactic radiosurgery (SRS) of brain tumors. Additionally, the feasibility of head rotation in both pitch and yaw directions was assessed based on previous studies on human head and neck motion ranges, which were primarily conducted in the context of rehabilitation research (14). Figure 1D illustrates the stabilization of the phantom head using a thermoplastic mask, as well as the adjustment of head angles with the base plate.
Before imaging and treatment, a 3-point thermoplastic mask was used to secure and immobilize the phantom head, attaching it firmly to the base plate. High-resolution CT imaging was performed using a Siemens Emotion 16 CT scanner with a 3 mm slice thickness. The images were then reconstructed to achieve a 1 mm slice thickness to ensure anatomical accuracy and enable detailed treatment planning.
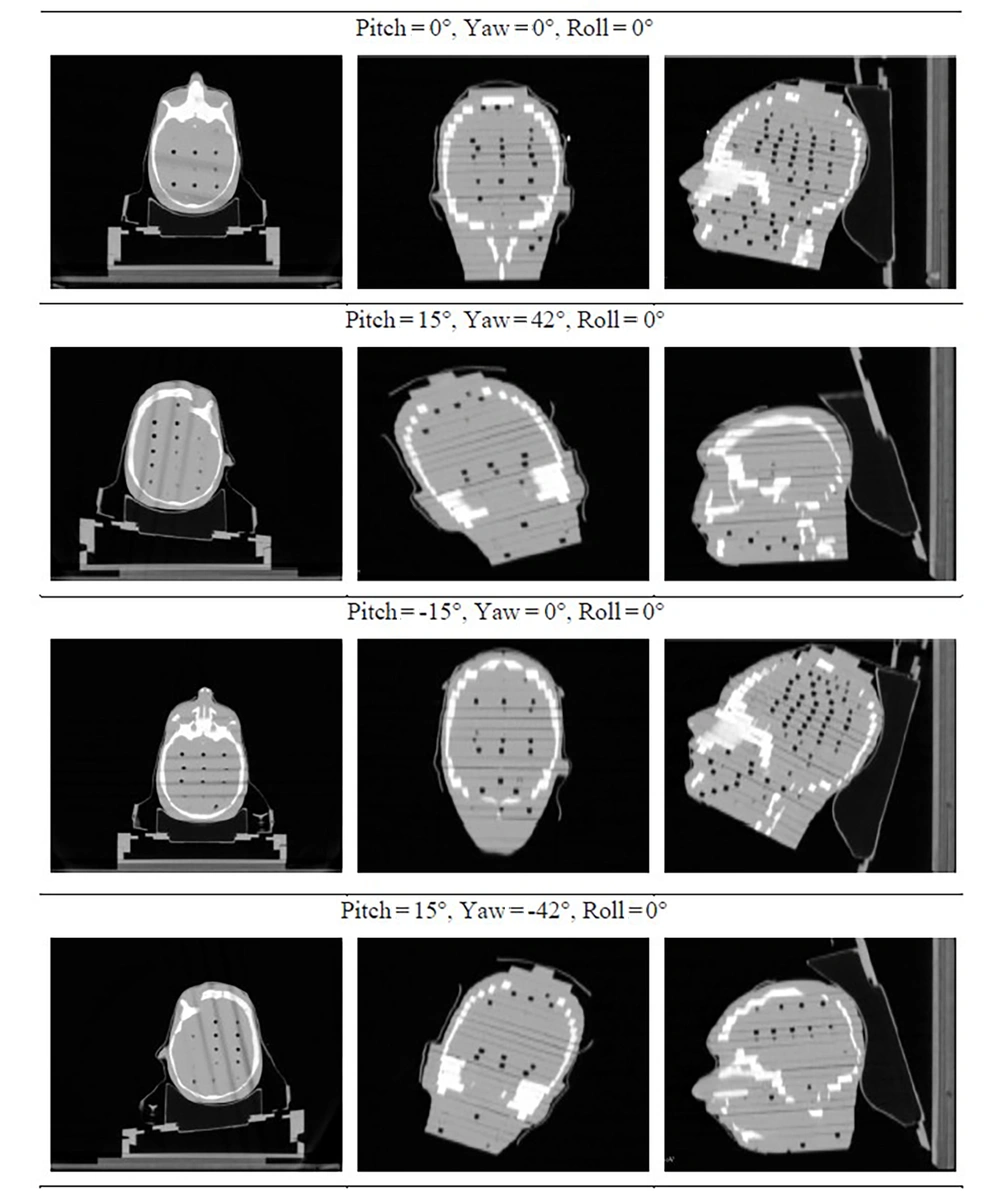
To simulate a range of clinical scenarios, the phantom was positioned in 4 distinct orientations:
1. Pitch = 0°, yaw = 0°, roll = 0°.
2. Pitch = -15°, yaw = 0°, roll = 0°.
3. Pitch = 15°, yaw = -42°, roll = 0°.
4. Pitch = 15°, yaw = 42°, roll = 0°.
These configurations were selected to represent diverse anatomical setups and to evaluate the potential benefits of noncoplanar beam arrangements. Figure 2 illustrates sample slices — in axial, coronal, and sagittal views — for each of the four configurations.
3.2. Organ Delineation and Treatment Planning
CT images were imported into the Precision treatment planning software (version 3.2, Accuray). An experienced radiation oncologist delineated the pseudo-hypophysis clinical target volume (CTV) and the nearby OARs, including the brainstem, optic chiasma, left and right optic nerves, eyes, and lens — based on the CT scan acquired in the neutral position (i.e., pitch = 0°, roll = 0°, and yaw = 0°). A 5 mm margin was added to the CTV to ensure target coverage and to define the planning target volume (PTV). In addition, a 3 mm margin was applied to account for the planning risk volume (PRV) of serial organs such as the optic nerves, chiasma, and brainstem. Two treatment planning scenarios were developed, one for the coplanar setting and the other for the noncoplanar one.
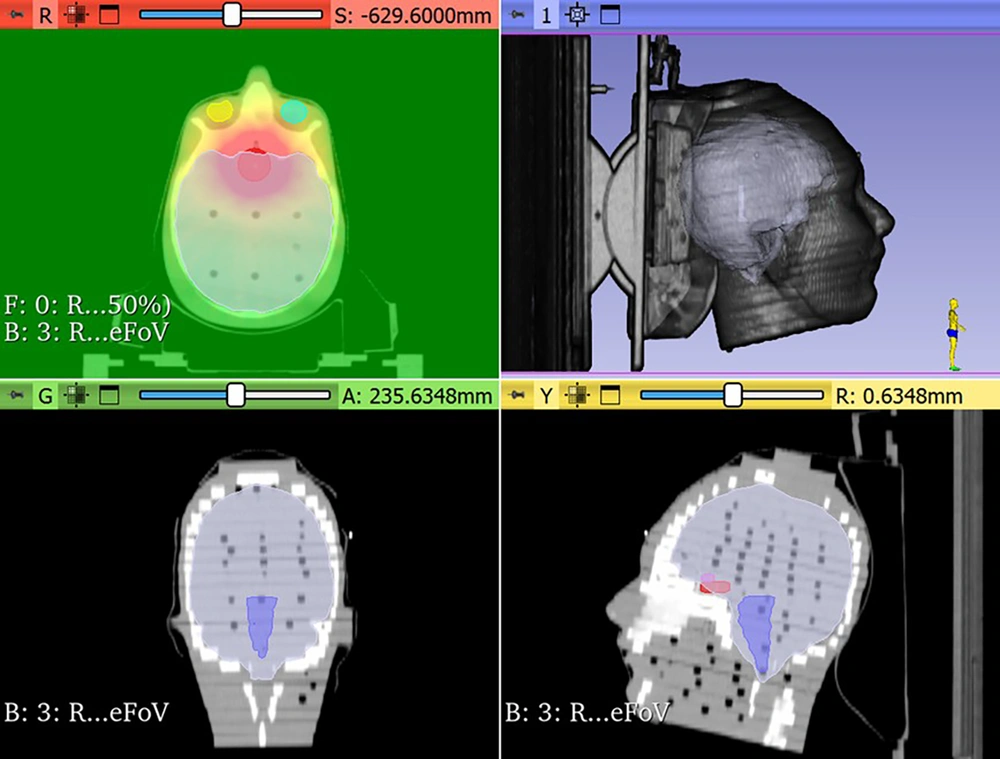
For the coplanar scenario, the prescription dose was 54 Gy to the PTV for the CT series acquired with no rotation. Treatment plans employed a 6 MV flattening filter-free (FFF) photon beam from the Radixact system, using a 2 mm dose calculation grid. The IMRT was the treatment technique applied in all cases. The planning goal was to cover 95% of the PTV with an isodose of 95% of the prescribed dose and a minimal dose of the defined OARs. The dose constraints for the OARs were as follows: Dmax < 54 Gy for the brainstem, optic chiasm, and both optic nerves; Dmax < 60 Gy for their PRVs; and Dmax < 60 Gy for the eyes. Some of the delineated organs, the PTV, and the color-washed dose distribution from a treatment plan are illustrated in Figure 3 for insight and clarification.
All 4 CT series were considered for the noncoplanar scenario, with the 54 Gy prescription dose evenly distributed among 4 hypothetical noncoplanar beams, each delivering 13.5 Gy to the target. As in the coplanar scenario, the planning objective was to cover 95% of the PTV with an isodose corresponding to 95% of the prescribed dose (13.5 Gy in this case). Target volumes and OARs were transferred from the original neutral CT series to the tilted configurations using the PresiceRTX® module of Precision TPS. Separate plans were generated for each configuration.
All RTimage, RTDose, and RTStructure files from the generated plans were exported as DICOM files and imported into 3D Slicer (Version 5.0.3), an open-source image analysis and visualization platform. The software supports multimodal imaging and enables precise dose assessment through customizable modules and Python scripting. The cumulative dose to the target volume and OARs, as well as the resulting dose-volume histogram (DVH) parameters, were analyzed by registering the 4 CT datasets with their respective radiation therapy dose.
A comparison was performed between the coplanar scenario, in which the full dose was delivered to a single neutral-position CT dataset, and the noncoplanar scenario, which incorporated multiple head orientations.
3.3. Conformity and Homogeneity Indices Calculation
To quantitatively assess the dosimetric differences between the coplanar and noncoplanar techniques, the Paddick Conformity Index (PCI) and the Homogeneity Index (HI) were calculated (10, 11).
The PCI was calculated using Equation 1:
Where VT,ref is the volume of the target (PTV) receiving at least the reference isodose (95% of the prescribed dose), VT is the total volume of the PTV, and Vref is the total volume of the reference isodose (95%) (12).
The HI was determined using Equation 2:
Where D2% is the dose received by 2% of the PTV (indicating the highest dose region), D98% is the dose received by 98% of the PTV (indicating the lowest dose region), and D50% is the dose received by 50% of the PTV (median dose). These indices were calculated for both the coplanar and noncoplanar treatment plans to evaluate dose conformity and homogeneity. The required volumetric and dosimetric data were extracted from the DVH of each plan.
4. Results
The registration of CT images using 3D Slicer software enabled the generation and analysis of noncoplanar treatment plans. This analysis evaluated the impact of different beam arrangements on target coverage and OARs sparing, offering insights into the potential advantages of noncoplanar delivery.
Volumetric dose histograms revealed clear dosimetric advantages of noncoplanar radiation therapy. Table 1 summarizes the mean and maximum doses to OARs and target coverage for both scenarios. As shown, the noncoplanar therapy demonstrated significant notable reductions in maximum doses to the visual apparatus, including the right eye (-2.2 Gy), left eye (-2.6 Gy), and left optic nerve (-3.9 Gy). Both globe lenses exhibited the greatest dose reduction, of approximately 6 Gy, when shifting to the noncoplanar setup. However, no significant differences in maximum doses were observed for the chiasma and brainstem.
| Structure | Coplanar (Gy) | Noncoplanar (Gy) | Relative Difference (%) a | |||
|---|---|---|---|---|---|---|
| Mean Dose | Max Dose | Mean Dose | Max Dose | Mean Dose | Max Dose | |
| Optic chiasm | 46.7 | 53.5 | 46.5 | 53.5 | -0.4 | 0.0 |
| Brainstem | 1.9 | 19.2 | 1.9 | 18.9 | -0.5 | -1.6 |
| Right eye | 9.9 | 19.7 | 9.5 | 17.5 | -4.2 | -12.6 |
| Left eye | 9.7 | 18.6 | 9.4 | 16.0 | -3.2 | -16.3 |
| Right optic nerve | 15.8 | 39.8 | 15.2 | 39.7 | -3.9 | -0.3 |
| Left optic nerve | 14.6 | 30.9 | 14.4 | 27.0 | -1.4 | -14.4 |
| Right lens | 12.1 | 13.3 | 6.9 | 7.1 | -75.7 | -87.9 |
| Left lens | 11.3 | 12.6 | 6.1 | 6.9 | -84.4 | -82.6 |
a Relative difference (%) = (Noncoplanar - coplanar)/Noncoplanar × 100.
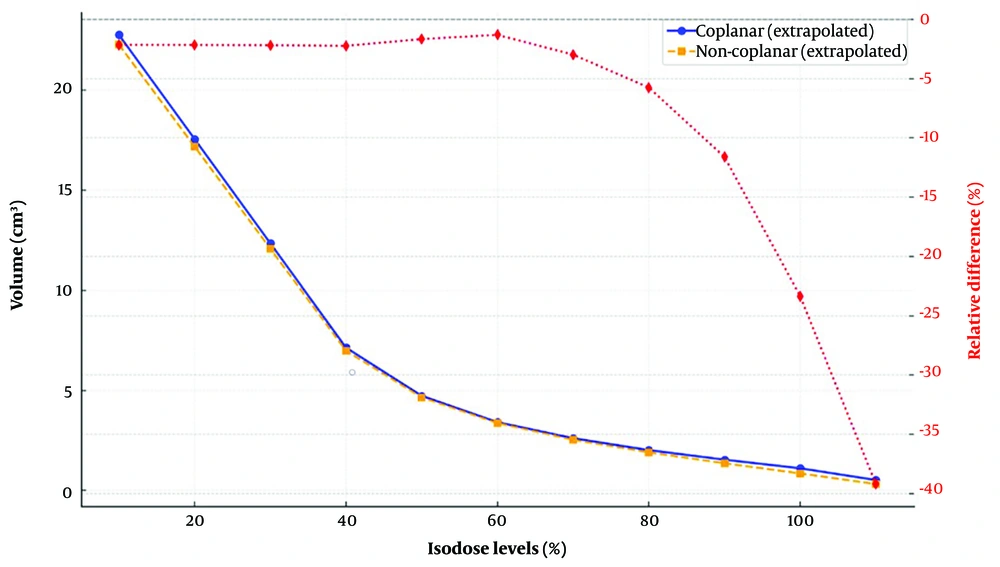
To further substantiate the observed improvements in OARs sparing with the noncoplanar technique, a paired t-test was performed to compare mean and maximum dose values for each OARs between the two delivery methods. Statistically significant reductions (P < 0.05) were observed in several critical structures, particularly the eyes, lenses, and optic nerves. The lenses exhibited the greatest benefit, with substantial dose reductions, followed by the optic nerves — both of which are highly radiosensitive. These findings are clinically relevant, as excessive radiation to these structures are associated with permanent visual impairment and complications such as cataract formation. Notably, these dosimetric gains were achieved without compromising target coverage. The isodose volume comparisons between coplanar and noncoplanar approaches are presented in Figure 4. This figure visually illustrates the volumetric differences between the two techniques, along with their relative percentage changes.
The pseudo-CTV volume was approximately 5.3 cm3, and the PTV volume was 20.7 cm3. Dose DVH parameters further support the advantages of noncoplanar radiation therapy. For the noncoplanar techniques, D95%, D97%, and D98% values were 56.1 Gy, 57.3 Gy, and 57.9 Gy, respectively, whereas the corresponding values for the coplanar technique were 52.8 Gy, 53.9 Gy, and 54.5 Gy. These results indicate improved dose coverage and homogeneity for the target volume when using noncoplanar setups.
The PCI was 0.728 for the coplanar treatment and increased to 0.902 for the noncoplanar approach, indicating a substantial improvement in dose conformity. These values are consistent with previously reported PCI values for coplanar tomotherapy treatments, which typically range from 0.65 to 0.78, confirming that the PCI obtained in this study falls within the expected range for this technique. The observed increase in PCI for the noncoplanar plan underscores its capability to achieve superior dose conformity while maintaining optimal normal tissue sparing.
Similarly, HI was 0.094 for the coplanar plan and 0.031 for the noncoplanar plan, demonstrating a substantial reduction in dose heterogeneity with the noncoplanar approach. A lower HI value indicates more uniform dose distribution within the PTV, thereby reducing dose hotspots and underdosed regions — an important factor in improving tumor control and minimizing treatment-related complications.
5. Discussion
This study investigates the use of noncoplanar beams in tomotherapy for brain tumor treatment through a novel head base plate that permits controlled pitch and yaw adjustments. By comparing dosimetric outcomes between coplanar and noncoplanar techniques in a phantom model, the results demonstrate that the noncoplanar tomotherapy can improve dose conformity, reduce radiation exposure to critical organs, and improve treatment precision for brain tumors.
The results demonstrate significant reductions in the maximum dose to critical visual structures with noncoplanar tomotherapy. These reductions, which range from 0 to 6.2 Gy, suggest a substantial improvement in sparing OARs. No difference was observed in the maximum dose to the optic chiasm, which may be attributed to its partial overlap with the PTV.
Based on the data presented in Table 1 and Figure 4, noncoplanar techniques exhibited higher conformity and reduced low-dose radiation spread. By delivering radiation with a more conformal dose distribution, this approach minimized exposure to surrounding healthy tissues. This reduction in low-dose spread has important clinical implications, including lower risks of normal tissue toxicity and secondary malignancies, as well as improved cosmetic outcomes in sensitive regions such as the head and neck.
Our findings are consistent with those of Yuasa and Kurosaki, who also demonstrated the feasibility and dosimetric advantages of noncoplanar radiation in tomotherapy. While their study employed a tilt-type head and neck fixture in pitch direction combined with deformable image registration (DIR) for dose tracking, our approach integrates a simpler yet effective mechanical solution that also enables head tilting in the yaw direction. Furthermore, unlike their emphasis on dose distribution within spherical PTVs, our study focuses on critical visual structures. Both studies, however, corroborate that noncoplanar radiation reduces low-dose spread compared to coplanar methods, thereby addressing concerns regarding the "low-dose bath" associated with tomotherapy (13).
Reducing radiation dose to critical structures such as the optic nerves has important clinical implications, as it may lower the risk of long-term complications, including radiation-induced optic neuropathy — a severe and often irreversible condition. Additionally, minimizing radiation exposure to the lenses and eyes reduces the likelihood of vision impairment and cataract formation.
Furthermore, the improved dose distribution achieved with the noncoplanar approach may allow clinicians to escalate the tumor dose, thereby enhancing local control while maintaining patient safety (15). Additionally, the improved dose-volume ratio observed in noncoplanar plans indicates superior dose conformity to the target while minimizing exposure to surrounding healthy tissues. This is consistent with the objectives of modern radiation therapy techniques, such as SRS, which aim at achieving high therapeutic efficacy while reducing adverse effects in the treatment of brain tumors or metastases (16).
Importantly, the reduced low-dose distribution associated with noncoplanar beams may lower the risks of secondary malignancies, which is particularly relevant for younger patients or those with longer life expectancies (17). This aligns with the broader objectives of personalized radiation therapy, in which treatments are tailored to maximize therapeutic benefits while minimizing associated risks. Other dedicated radiotherapy systems, such as GammaKnife and CyberKnife, have demonstrated significant dosimetric advantages in stereotactic treatments; however, these modalities often require specialized equipment and complex workflows. By contrast, our approach adapts standard tomotherapy systems through the use of a novel, cost-effective base plate. These findings contribute to the growing body of evidence supporting the potential of noncoplanar techniques to bridge the gap between improved OARs sparing and enhanced target coverage.
Additionally, the improved dose-volume ratio observed in noncoplanar plans indicates superior dose conformity to the target while minimizing exposure to surrounding healthy tissues. This is consistent with the objectives of modern radiation therapy techniques, such as SRS, which aim at achieving high therapeutic efficacy while reducing adverse effects in the treatment of brain tumors or metastases (16). The improvements in PCI and HI observed in this study indicate that the novel noncoplanar base plate offers an effective approach for enhancing tomotherapy delivery, enabling superior target coverage while improving OARs sparing. Future research should focus on clinical validation of these findings, including patient-based studies to further assess the feasibility and potential benefits of noncoplanar tomotherapy in real-world clinical settings.
The calculated conformity and homogeneity indices further underscore the advantages of implementing a noncoplanar technique in tomotherapy. The PCI values obtained in this study are consistent with prior reports on tomotherapy-based treatments, such as those of Thakur et al., in which coplanar PCI values typically range between 0.65 and 0.78 (18). This consistency supports the robustness of the treatment planning approach employed in our work. The higher PCI observed in the noncoplanar setup (0.902) reflects superior dose conformity, which is critical for minimizing unnecessary irradiation to surrounding healthy tissues while maintaining effective tumor coverage.
The Inferior Conformity Index of tomotherapy compared to other techniques, such as RapidArc, in the treatment of benign intracranial tumors has been reported by previous researchers, including Fogliata et al (19). Similarly, Audet et al. in their study on cranial radiosurgery using VMAT, concluded that for cranial targets with a diameter greater than 7 mm, noncoplanar arcs offer more accurate and conformal dose distributions while delivering lower doses to healthy tissues (20). Furthermore, the lower HI (0.031) observed in the noncoplanar plan suggests a more homogeneous dose distribution, reducing dose heterogeneity within the PTV. This reduction in dose hotspots can lower the likelihood of radiation-induced toxicity, while avoiding underdosed regions helps ensure effective tumor control.
The improvements in PCI and HI observed in this study indicate that the novel noncoplanar base plate offers an effective approach for enhancing tomotherapy delivery, enabling superior target coverage while improving OARs sparing. Future research should focus on clinical validation of these findings, including patient-based studies to further assess the feasibility and potential benefits of noncoplanar tomotherapy in real-world clinical settings.
This study introduces an innovative mechanical solution for integrating noncoplanar capabilities into tomotherapy systems, demonstrating both its feasibility and dosimetric advantages. However, several limitations should be acknowledged. First, the study was conducted using a phantom model, and the results may not fully reflect clinical performance. Variations in patient anatomy, tumor location, and motion during treatment could influence the outcomes. Second, although the observed reduction in dose to critical organs is promising, further investigation is needed to determine its clinical significance, particularly in relation to long-term patient outcomes such as reduced toxicity and improved quality of life.
Finally, the mechanical adjustments introduced by the base plate may present additional challenges in treatment setup and immobilization, and potentially increase the overall treatment time. These issues should be addressed in future studies, for example, by incorporating electronic capabilities for automated base plate adjustments.
Assuming evenly distributed weights for each configuration may limit the full potential. Therefore, the results reported here could be further improved by applying inverse planning with optimized field weight adjustments. This suggests that the results reported here may underestimate the true potential of our base plate. The present work can be regarded as a feasibility study intended to encourage future integration of this capability into tomotherapy planning software optimization algorithms. However, a notable drawback of this technique is the potential to slow down the treatment procedure and delivery, as a separate delivery pass is required for each configuration and plate adjustment, which may also necessitate an additional IGRT session for verification. Building on these findings, future research should aim at validating the technique in clinical settings across diverse patient populations. Prospective clinical trials could assess the efficacy of noncoplanar tomotherapy in reducing treatment-related toxicities and enhancing tumor control. Additionally, the integration of DIR and adaptive planning into tomotherapy systems could further enhance the precision and safety of noncoplanar treatments. Exploring applications of this technique in other anatomical sites, such as the brain, spine, and thorax, may broaden its utility and enhance its clinical impact.
5.1. Conclusions
This study presents a novel head base plate enabling noncoplanar tomotherapy, demonstrating significant dosimetric improvements in both dose conformity and OARs pairing. By addressing a critical limitation of tomotherapy systems, this innovation has the potential to improve treatment precision and enhance patient safety in brain radiotherapy. The improved conformity and homogeneity indices observed with noncoplanar setups suggest that this approach can optimize dose delivery while minimizing radiation exposure to critical structures. These findings are consistent with ongoing advancements in radiotherapy techniques aimed at improving therapeutic outcomes and reducing long-term complications. Moreover, further refinement of treatment planning algorithms and integration into clinical workflows could enhance the practical implementation of this technique. If validated in clinical practice, noncoplanar tomotherapy could become a valuable addition to modern radiotherapy approaches, potentially improving patient outcomes across various tumor sites. By extending this method to other anatomical regions, such as the brain and spine, the potential benefits of noncoplanar delivery could be further leveraged, paving the way for broader clinical applications and establishing new standards in radiation therapy.
5.2. Limitations
This study was conducted entirely using a phantom model to evaluate the feasibility and dosimetric benefits of the proposed noncoplanar base plate for tomotherapy. While the phantom setup allowed for precise control of variables and reproducibility, the findings may not fully reflect the complexity of clinical scenarios, including variations in patient anatomy, tumor location, and intra-/inter-fraction motion. Therefore, clinical validation through patient-based studies is necessary to confirm the feasibility, safety, and potential therapeutic advantages of this approach. Such studies should also investigate patient comfort, immobilization accuracy, and workflow integration in real-world settings.
Also, the total dose (54 Gy) was equally divided between the 4 head positions (13.5 Gy each) to keep the conditions simple and facilitate a direct comparison between coplanar and noncoplanar setups in the current study. The primary goal was to isolate the effect of beam angle changes without introducing additional variables from differing dose weights. Furthermore, the dose division approach was based on methodologies used in Yuasa et al.’s study employed a similar equal dose distribution strategy in their phantom study on noncoplanar radiation using tomotherapy (13). To avoid introducing further variables in this preliminary investigation, the same approach was adopted here. However, it is clear that for clinical implementation, this variable is critical. Therefore, instead of simply dividing the dose equally, optimizing the dose distribution among positions is essential to achieve an optimal treatment plan.