1. Background
Carcinogens are substances that are directly responsible for cell damage or strengthening and helping to cause cancer. Among the carcinogens that cause lung tissue damage are tobacco and its components. Reports in this regard show that smoking is the main cause of lung disorders in 80% of men and 50% of women (1). While the relationship between cigarette smoke and lung tissue damage is mostly accepted, the effective mechanisms involved in the molecular cellular disorder of lung tissue have not been fully defined. Tobacco contains a variety of carcinogens, among which nicotine-derived nitrosamine ketone (NNK) is one of the strongest carcinogens and has a significant relationship with lung cancer (2, 3).
Carcinogens such as NNK can exert their biological effects in human cells and animal models and cause the activation of various inflammatory cascade signaling pathways in different body organs, including lung tissue (4). One of the most important factors of the inflammatory signaling pathways is the transcriptional message transducer and activator gene-3, which, through STAT3 signaling, causes the expression of various genes in the downward cascade path, such as cell cycle genes (5). After being phosphorylated and transferred from the cytoplasm to the nucleus, this gene can cause the transcription of genes involved in cell proliferation and survival (6). Either it is directly induced through tobacco carcinogens and their components and activates the tumorigenesis pathways (7) or it does not have a stimulating role on the activity of the pathways leading to the tumor due to the induction of NNK (4). The tobacco-specific carcinogen NNK promotes lung tumorigenesis by establishing a synergistic crosstalk between the angiotensin II receptor type 1 (AGTR1) and IGF-1R/insulin receptor (IR) signaling pathways. Mechanistically, NNK binding to the nAChR activates the Src/STAT3 axis, leading to the transcriptional upregulation of both angiotensinogen (AGT) and insulin-like growth factor 2 (IGF2).
While AGT initiates the AGTR1 pathway, the subsequent activation of AGTR1 facilitates the release of the STAT3-produced IGF2 via PLC-intervened calcium release. This molecular cooperation between the AGTR1 and IGF-1R/IR signals ultimately drives enhanced tumorigenic activity in lung epithelial and stromal cells (2). Also, it may change slightly after being affected by carcinogens, but it does not affect the activation of these pathways leading to tumor (8). It seems that the increased activity of this transcription factor in physiological (9) and pathological (7) conditions indicates the different functions of this transcription factor in growth conditions in healthy tissue and tissues exposed to carcinogens. Therefore, investigating the role of this transcription factor in healthy tissue exposed to the NNK carcinogen needs further investigation. On the other hand, given STAT3's crucial role in cancer development, blocking STAT3, through either genetic or drug-based methods, prevents the viability and growth of malignant cells across numerous experimental setups (10). In this regard, research on the anti-cancer effects of Nigella sativa (black seed) shows mixed results. α-hederin, a key compound, demonstrated direct antitumor activity against Lewis Lung carcinoma in mice. Additionally, dietary supplementation with N. sativa offered a broad protective effect against chemical-induced carcinogenesis, inflammation, and oxidative stress in the lung, skin, and colon. However, contrasting in vitro data reported that α-hederin and thymoquinone (TQ) failed to enhance cytotoxicity or apoptosis in specific human lung (A549) and larynx (HEp-2) cancer cell lines (11).
Moreover, various factors aid in mitigating issues arising from the impact of carcinogens, including physical activity. Certain studies suggest the benefits of physical activity for cancer patients throughout their treatment and recovery. Just one bout of physical activity triggers molecular signaling pathways in muscles pertinent to energy metabolism, and the impact of these on metabolic health has been observed. In addition, the continuation of training can also lead to useful metabolic adaptations, and these acute responses and chronic adaptations in physical activities can improve the metabolic function of muscles in cancer patients (12). Sports activity can also cause the physiological restructuring of the organs. Subject to the particular exercise, multiple elements and distinct signaling pathways orchestrate this restructuring, which mandates adjusting the activation of expression for many genes responsible for the structural integrity and operational capacity of that organ (13).
Despite numerous studies on how exercise training impacts tumor-promoting pathways, further investigation is required into the combined influence of physical activity and environmental carcinogen-induced pathway activation, particularly with NNK. The use of herbal supplements represents a method to gain advantages, especially when coupled with submaximal sports activities, to diminish inflammatory markers in individuals exposed to environmental carcinogens. In this case, exercise training has been demonstrated to prevent cancer risks and immune issues like lung inflammation. Regarding the exercise-related modalities, submaximal endurance swimming and herbal supplements with fewer drug adverse effects are equally crucial. In this regard, Testa et al. (14) demonstrated that elevated plasma interleukin-6 (IL-6) levels as well as oxidative damage within skeletal muscle led to STAT3 activation, which is a major contributor to tumor-related muscle wasting.
Notably, regular exercise training mitigated muscle degeneration by blocking STAT3 phosphorylation, which consequently prevented the decline in IL-6 and the peroxidation of muscle lipids. The defense mechanism assisted in inhibiting the proliferation of crucial genes and proteins of the ubiquitin-proteasome and autophagy pathways in mice with tumors, for instance, Atrogin-1, LC3B-II, Beclin-1, and p62. Consequently, it is expected that performing regular submaximal swimming exercises along with herbal supplements that have a common border in the inflammatory signaling pathway may have more effective responses on inflammatory processes. Nigella sativa (black seed or black cumin) is among the herbal supplements that seem to have well-known therapeutic effects in various inflammations and cancers. In this study, it was shown that the nano form of this plant is more effective than its ethanol form in reducing inflammation and pathways leading to tumors (15).
2. Objectives
No research has been found on the effect of black seed nanocapsule on the pathway leading to tumor in STAT3 signaling; Although some studies have investigated the anti-cancer effects and cell cycle inhibition of black seed and TQ in cancer tumors (16, 17). However, investigating the anti-inflammatory response of black seed nanocapsule and its protective effects on the pathways leading to tumor creation through NNK on the one hand and the use of regular submaximal swimming exercises on the other hand requires further study. Thus, this paper aimed at investigating the effect of 12 weeks of swimming training and injection of black seed nanocapsules on the expression of the STAT3 gene in the lungs of rats following exposure to NNK carcinogen.
3. Methods
3.1. Study Design, Ethics, and Animal Housing
3.1.1. Study Design
This investigation employed a randomized, controlled experimental design to determine the effects of swimming exercise and black seed nanocapsule supplementation on the expression of the STAT3 gene in the lungs of Wistar rats following NNK administration.
3.1.2. Ethical Compliance and Housing
All animal research methods and procedures adhered to the endorsements of AAALAC and were in agreement with European Union rules (DC 86/609/EEC, 2003/65/EC, 2010/63/EU). This study is approved under the ethical approval code of MUBABOL.HRI.REC.1395.10. A total of 86 adult male Wistar rats (average weight: 103.84 ± 27.93 grams) were acquired from the Pasteur Research Institute. The animals were housed individually in transparent polycarbonate cages (4 rats per cage for physiological compatibility) under controlled laboratory conditions: 22 ± 1.4 degrees Celsius, relative humidity of 55 ± 5%, and a 12-hour light-dark cycle (light from 6 AM to 6 PM). Standard pellet food and water were provided ad libitum.
3.2. Animal Preparation and Group Randomization
3.2.1. Acclimatization and Familiarization Period (2 Weeks)
1. Week 1: Rats were acclimatized to the new laboratory environment.
2. Week 2 (swimming familiarization): To reduce swimming stress and ensure adaptation, rats were individually placed in the water pool for 10 to 30 minutes daily, following the protocol by Mirdar et al. (18).
3.2.2. Randomization and Group Assignment
Following the 2-week preparation period, the 86 rats were randomly divided into 9 experimental and control groups (groups C, V, E, N, S, E.N, S.N, S.E, and S.N.E). The allocation was as follows: Eight groups of 10 rats each, and 1 control group (C) of 6 rats (Table 1).
| Group Abbreviation | Group Description | Intervention |
|---|---|---|
| C (n = 6) | Control | None (distilled water + No exercise) |
| V (n = 10) | Vehicle | Distilled water injection + No exercise |
| E (n = 10) | Exercise | Swimming training + Vehicle injection |
| N (n = 10) | NNK | NNK injection + Vehicle injection |
| S (n = 10) | Supplement | Supplement injection + Vehicle injection |
| E.N (n = 10) | Exercise + NNK | Swimming training + NNK injection |
| S.N (n = 10) | Supplement + NNK | Supplement injection + NNK injection |
| S.E (n = 10) | Supplement + Exercise | Supplement injection + Swimming training |
| S.N.E (n = 10) | Supplement + NNK + Exercise | Supplement injection + NNK injection + Swimming training |
Abbreviation: NNK, nicotine-derived nitrosamine ketone.
3.3. Detailed Intervention Protocols (12 Weeks)
All interventions (NNK, supplement, and exercise) were performed concurrently for 12 weeks.
3.3.1. Nicotine-Derived Nitrosamine Ketone and Black Seed Nanocapsule Administration Protocol
The NNK was injected subcutaneously in the groups, including NNK once a week, at the rate of 12.5 mg/kg of body weight for 12 weeks, and the solvent group was given distilled water made from the black seed plant material, which was prepared and identified by a medicinal plant store in Babolsar. After confirming the seed variety of the black seed plant by the herbarium expert of Mazandaran University, a sample of it was kept in the sports physiology laboratory. After preparation of black seed nanocapsules, this supplement was injected subcutaneously into the groups included in the supplement once a week at the rate of 125 μg/kg of body weight (19). Since the nano form of black seed, unlike black seed, does not cause toxicity in different doses, it can be used in the treatment of pathological conditions such as types of cancers and various pathogenic agents. In this study, the injection of 10 to 50 micrograms of black seed nanoparticles was compared with the injection of 100 micrograms of black seed oil in A549 lung cancer cells. The results showed that different doses of low nano have a significant difference from the dose of 100 micrograms of black seed oil in reducing lung cancer cells. MTT evaluation showed that the closer the nano dose is to 50 μg, the greater the reduction is seen in lung cancer cells (19). In this research, for the first time, a dose of 125 μg/kg of the rats' weight was used.
3.3.2. Progressive Swimming Training Protocol
Swimming training was also done in the groups that included training once a day (5 days a week), in a water tank with dimensions of 50 × 50 × 100 cm, with a temperature of 30 to 32 degrees, for 3 months. Training overload was done by adjusting the water speed and power (using a water speedometer and adjustment lever) while swimming. The duration of training in water after acclimatization to water on the first day of training was 25 minutes, with a weekly increase of 5 minutes. This duration reached 60 minutes in the 8th week, and then this time was stabilized and continued until the end of the 12th week. The water speed also started from 4 L/min and continued up to 10 L/min until the end of the training protocol (18).
3.4. Tissue Harvesting and Laboratory Procedures
3.4.1. Sample Collection
Forty-eight hours after the last training session and NNK/Vehicle injection, the rats were euthanized following anesthesia with a xylazine and ketamine solution. The lung tissue was immediately harvested, flash-frozen in liquid nitrogen, and stored at -70°C for subsequent gene expression analysis.
3.4.2. RNA Extraction and cDNA Synthesis
The continuation of the RNA extraction process was sent to the laboratory. RNA extraction steps were performed based on the Trizol protocol; the 100 Reaction K1622 kit instructions were used to synthesize STAT3 gene cDNA. First, the optimal concentration of cDNA was determined using a serial concentration test.
3.4.3. Real-time Polymerase Chain Reaction
Real-time PCR cycles for the STAT3 gene were run on a real-time PCR-ABI thermal program using 2 temperatures: 95°C and 60°C. Moreover, GAPDH was used as the reference (housekeeping) gene for normalization (Table 2).
| Genes | Primer Forward | Primer Reverse | NCBI |
|---|---|---|---|
| STAT3 | ACCAACGACCTGCAGCAATA | ACACTCCGAGGTCAGATCCA | ID25125 |
| GAPDH | AAGTTCAACGGCACAGTCAAGG | CATACTCAGCACCAGCATCACC | ID24383 |
3.5. Statistical Analysis
The study data were analyzed using SPSS-19 and Excel software. The normality of the variable distribution across different groups was assessed using the Kolmogorov-Smirnov test. Due to the normality of the data, the parametric test of one-way analysis of variance (ANOVA) was used to compare the means across the multiple groups. Tukey's post-hoc test was employed for pairwise comparison of the data following a significant ANOVA result. Statistical significance was accepted at a level of P < 0.05. Data were described using descriptive statistics, and graphs were created using Excel software.
4. Results
The descriptive findings of the current research are shown in Table 3. In the current study, we assessed the initial weight of rats to check the normality of distribution across different groups, and our results demonstrated that there was no significant difference between the initial weight of the study’s groups (P < 0.05).
| Groups Variables | Control | Solvent | NNK | Supplement + NNK | Exercise + NNK |
|---|---|---|---|---|---|
| Initial weight (g) | 102.5 ± 12.8 | 103.6 ± 10.2 | 106.7± 20.1 | 102.4 ± 21.4 | 111.3 ± 36.5 |
| Final weight (g) | 2495.5 ± 23.8 | 259.5 ± 39.3 | 265.6 ± 70.4 | 270.2 ± 62.2 | 274.3 ± 61.8 |
| Height (cm) | 20.6 ± 23.8 | 20.9 ± 1.5 | 22.7 ± 1.4 | 22.4 ± 1.2 | 22.1 ± 1.3 |
| Lung weight (g) | 1.1 ± 0.1 | 1.3 ± 0.1 | 1.6 ± 0.2 | 1.8 ± 0.2 | 1.7 ± 0.4 |
| lung volume (mL) | 0.9 ± 0.2 | 1 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.2 | 1.1 ± 0.4 |
Abbreviation: NNK, nicotine-derived nitrosamine ketone.
a Values are expressed as mean ± SD.
The results of this research showed a significant difference between the mean expression of the STAT3 gene in the study groups (Sig. = 0.001, P < 0.05, F = 5.503; Table 4).
| Sources Change | Sum of Squares | Degrees of Freedom | Mean Square (Variance) | F | P | Effect Size |
|---|---|---|---|---|---|---|
| Between groups | 1743.2 | 8 | 217.91 | 5.503 | 0.001 | 0.37 |
| Within groups (error) | 3009.4 | 76 | 39.59 | |||
| Total | 4752.7 | 84 | - |
The results of Tukey's post-hoc test also showed that the level of STAT3 gene expression between the training group and the control group (P = 0.986), as well as the supplement and solvent group (P = 0.991), was not significant (P < 0.05). Also, in comparison between the group of exercise-NNK with solvent (P = 1.000), supplement-NNK with solvent (P = 1.000), and exercise-supplement-NNK with solvent (P = 0.990), there was a significant difference (Table 5). The comparison between the two groups of exercise with NNK-exercise (P = 0.980) and supplement with NNK-supplement (P = 0.929) showed no significant difference between these groups (P > 0.05).
| Groups and Comparison | Standard Error | P | Effect Size |
|---|---|---|---|
| NNK | |||
| Solvent | 2.814 | 0.002 a | 1.47 |
| Supplement-NNK | 2.615 | 0.001 a | 1.63 |
| Exercise-NNK | 2.318 | 0.002 a | 1.47 |
| Exercise-supplement-NNK | 2.258 | 0.0001 a | 1.84 |
| Exercise | |||
| Control | 3.249 | 0.986 | 0.007 |
| Exercise-NNK | 2.230 | 0.980 | 0.012 |
| Exercise-supplement-NNK | 2.031 | 1.00 | 0.00 |
| Supplement | |||
| Solvent | 2.622 | 0.991 | 0.005 |
| Supplement-NNK | 2.425 | 0.929 | 0.041 |
| Exercise-supplement-NNK | 2.270 | 1.00 | 0.00 |
| Exercise-NNK | |||
| Solvent | 2.962 | 1.00 | 0.00 |
| Exercise-supplement-NNK | 2.291 | 0.982 | 0.011 |
| Supplement-NNK | |||
| Solvent | 2.399 | 1.00 | 0.00 |
| Exercise-supplement-NNK | 2.001 | 0.932 | 0.038 |
| Exercise-supplement-NNK | |||
| Solvent | 2.021 | 0.990 | 0.007 |
Abbreviation: NNK, nicotine-derived nitrosamine ketone.
a Significant level at P < 0.05.
While comparing the level of STAT3 gene expression in the NNK group with the three groups of supplement-NNK (P = 0.0001), exercise-NNK (P = 0.002), and exercise-supplement-NNK (P = 0.0001) showed a significant increase in the expression of this gene in the NNK group compared to the mentioned groups (Table 5 , Figure 1).
And finally, a significant increase in the expression of the STAT3 gene was observed in the NNK group compared to the solvent group (P = 0.002) at a significant level of P < 0.05 (Table 5).
5. Discussion
Comparing the relative changes of the STAT3 gene in the lung tissue of Wistar rats through the swimming training illustrated no considerable difference compared to the control group. The findings of the current research showed that the implementation of aerobic swimming exercises does not cause a significant difference in the relative changes of the STAT3 gene in the lung tissue of the trained rats. Regarding the swimming training, it has been well-known that swimming offers protection against exercise-induced bronchoconstriction (EIB) primarily due to the warm and humid air found in indoor pools. This reduces the cold, dry air exposure that typically triggers EIB in other sports. Furthermore, swimming recruits actively and strengthens the respiratory muscles and improves the elasticity of the chest wall, contributing to superior long-term lung function (20). Moreover, several studies have illustrated that a 12-week aerobic program, such as submaximal swimming, bolsters respiratory muscle strength (like the diaphragm), boosting oxygen efficiency and reducing breathlessness. This approach is highly effective for improving function and quality of life in chronic conditions like chronic obstructive pulmonary disease (COPD) and asthma (21-23).
Research investigating the immediate impact of physical activity on healthy tissues has shown that skeletal muscle produces cytokines, specifically IL-6 as a myokine, which in turn stimulates STAT3 phosphorylation and its activity. It has been reported that acute aerobic exercise, by increasing myokines and activating the IL-6-STAT3 signaling pathway, contributes to the improvement of damaged peripheral nerves (24). It was also revealed that increased activity of the IL-6-STAT3 signaling pathway caused muscle hypertrophy in rats as a result of resistance training (25). The difference in the findings of this research and the above findings can be related to the type of exercises performed in the two studies. In this case, Ashour et al. (24) investigated the role of IL-6 and the STAT3 pathway in peripheral nerve injury (PNI) and the effect of exercise on nerve regeneration. They demonstrated that the combination of pre- and post-conditioning exercise showed better results than post-conditioning alone and concluded that exercise-induced IL-6 is valuable for nerve regeneration, and the IL-6/STAT3 pathway could be a therapeutic target for PNI (24). Furthermore, it has been illustrated that a single bout of exercise rapidly increased IL-6 and SOCS3 mRNAs alongside STAT1 and STAT3 phosphorylation, upregulating CyclinD1 and cMyc while downregulating MyoD and Myf5 (26). The second point is that the STAT3 pathway's activation is determined by the cytokine source. Since intense exercise activates STAT3 and can alter neutrophil genes, the resulting tissue damage may mimic a pathological inflammatory state, overriding the muscle's metabolic signaling. Therefore, pathological conditions in the tissue lead to an increase in this signaling pathway (27).
Regarding the exercise intervention, submaximal swimming (training + NNK) significantly decreased STAT3 gene expression in NNK-exposed rat lungs compared to NNK alone. Adding the supplement did not further enhance this STAT3 reduction. Thus, the study concludes that 12 weeks of swimming has an anti-inflammatory role by reducing STAT3 gene expression, suggesting low-intensity aerobic exercise may suppress the JAK-STAT3 pathway. Low-intensity aerobic exercise can be effective in reducing the asthma phenotype by changing the JAK-STAT signaling pathway in the airway epithelium (28). Hence, the JAK-STAT3 signaling pathway, which is activated by inflammation and increased IL-6 through macrophages, can be reduced through endurance exercise (29). The comparison of 60 minutes of swimming and running training in chronic kidney inflammation showed that the activity of the STAT3 oncogene is downregulated in both types of training. Although no significant difference was seen between the type of exercise and STAT3 reduction (30). On the other hand, it was shown in research that the number of nodules and abnormal hyperplastic cells in the lungs of rats in the group of aerobic and anaerobic exercises following the urethane carcinogen was significantly lower than the control group (31).
On the other hand, black seed nanocapsules (NNK + supplement) significantly decreased STAT3 phosphorylation in rat lung tissue exposed to NNK. However, exercise intervention (NNK + exercise + supplement) did not result in a statistically greater STAT3 reduction. In line with the findings of this research, some investigators demonstrated in their research that in allergic lung inflammation and rheumatoid arthritis, black seed and its components can improve the complications of these chronic conditions by blocking and suppressing pro-inflammatory factors (32, 33). Based on this, black seed nanocapsules can reduce STAT3 phosphorylation via IL-6 reduction. It also uses pathways like p53, MAPK, and PPARγ for its anti-tumor effects (34). Also, as a transcription factor, NF-κB can play a decisive role in various stages of tumor formation, including initiation, progression, invasion, and metastasis by forming chronic inflammatory responses (35). The activation of NF-κB by nicotine and NNK has been reported in lung cancer cells (36). In this regard, some reports show that the decrease in STAT3 phosphorylation due to black seed can be associated with the decrease in NF-κB activity (37). Because STAT3 can also maintain NF-κB (38). Therefore, it is possible that black seed nanocapsules combined with swimming effectively inhibit STAT3, linking NF-κB suppression to anti-inflammatory effects. In this regard, Sheikhnia et al. showed in a research that the combination of bortezomib with the use of TQ (the main component of black seed) inhibits NF-κB and STAT3 signaling in cancer cells by inhibiting proteasomes (39).
Moreover, it has been reported that black seed nanocapsules reduce STAT3 phosphorylation, possibly by inhibiting JAK kinases in cancer cells (39). Black seed nanocapsules may reduce STAT3 phosphorylation by lowering pro-inflammatory cytokines. Inhibiting STAT3 also downregulates anti-apoptosis genes like MCL-1 in cancer cells (40). Protein blocker of activated STAT (PIAS) is another mechanism that inhibits STAT3 through black seed. These blockers specifically interact with the activated STAT3 and, as a result, inhibit the activity of STAT3 through DNA binding and prevent the expression and induction of the STAT3 gene (41). Consequently, combining submaximal exercise and black seed nanocapsules helps counteract NNK carcinogens and inflammation via STAT3. This study's primary strength lies in its novel combinatorial approach, simultaneously investigating the effects of endurance exercise (swimming) and a specific nutritional supplement (black seed nanocapsules) on a crucial carcinogenic pathway. Using the NNK carcinogen provides a relevant model for tobacco-related lung cancer, and the inclusion of 8 comprehensive groups allowed for robust comparisons to isolate the specific effects of the training and the supplement. Furthermore, the use of nanocapsules represents a modern, targeted delivery strategy. However, a key limitation is the reliance on STAT3 gene expression as a surrogate marker. While significant, the 12-week duration is insufficient to observe long-term clinical endpoints, such as tumor count or overall survival, limiting direct translational certainty. Future studies must be of longer duration to assess these true carcinogenic outcomes. Additionally, the analysis of secondary morphological outcomes (e.g., lung weight) was descriptive; future research should include inferential statistics for these measures and expand to investigate other complementary inflammatory markers and signaling pathways to fully elucidate the mechanism of action. Finally, findings from the Wistar rat model require confirmation in human clinical trials.
5.1. Conclusions
The results of the present research generally show the effect of submaximal endurance activities and black seed nanocapsules against transcriptional message activator-3, which increases due to environmental carcinogens in the tissue environment, and training and black seed nanocapsule supplementation can partially inhibit this. The invoice should be transcribed. Based on this, regular exercise and natural supplements such as black seed nanocapsules can be mentioned as anti-inflammatory factors against all types of carcinogens. Also, supplements can have such anti-inflammatory properties that they can be used in appropriate doses and for a long time. The findings of this research state that regular aerobic exercise with black seed nanocapsules can be used as a complementary treatment method along with other lung cancer treatment methods; however, more studies are needed to better understand the molecular and cellular mechanisms involved in the beneficial effects of various types of exercise and supplements on lung tissue in lung cancer.
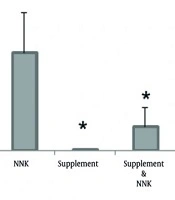
![Average expression levels of the STAT3 gene in research groups [values are based on mean ± SD; * significant level at P < 0.05 compared with nicotine-derived nitrosamine ketone (NNK)]. Average expression levels of the STAT3 gene in research groups [values are based on mean ± SD; * significant level at P < 0.05 compared with nicotine-derived nitrosamine ketone (NNK)].](https://services.brieflands.com/cdn/serve/31710/ae9283252a37cb56b55a3fcac022196a9ec9238d/ijcm-18-1-163669-i001-preview.webp)