1. Background
Secondary foramen oval defect is the most common form of atrial septal defect (ASD) and is associated with normal atrioventricular valves (AVs). Secondary ASD may be solitary or multiple, and foramina 2 cm or more in diameter are common in older children. Larger defects may extend downward into the inferior vena cava and coronary sinus foramen and upward into the superior vena cava or posteriorly (1-3). A person with a secondary foramen defect is often asymptomatic. The lesion may be discovered incidentally during a physical examination. Even a very large secondary ASD rarely causes clinical heart failure in children. In younger children, mild growth retardation and varying degrees of exercise intolerance may be observed. Often, the extent of the limitation may not be apparent to parents until after surgical repair, when growth or activity levels have significantly increased (1-3).
Chest radiographs show varying degrees of right ventricular (RV) and right atrial (RA) enlargement, depending on the size of the shunt. The pulmonary artery is enlarged, and the pulmonary vessels are dilated. These findings are variable and may not be noticed in mild cases. Cardiac enlargement is often best seen on the lateral view because the right ventricle bulges anteriorly as it increases in size (4).
Closure of ASD is recommended for all symptomatic patients. The time for non-emergency surgery is usually after the age of one year and before entering elementary school. Repair is best performed in early childhood because the mortality rate is much higher in young adults (3, 5). In general, primary ASD, sinus venosus, and coronary sinus defects require open-heart surgery, and only secondary ASD can be closed by intubation without open-heart surgery in certain circumstances (6-9).
In this method, the chest is usually opened through the middle of the sternum or through the left intercostal muscles to create access to the atria and the wall between them. The interatrial hole is then sutured and repaired with a patch. To ensure complete repair of the lesion, it is necessary to perform an echocardiogram at the end of the operation and before suturing the chest (9-11).
Regarding devices used through angiographic techniques, no detailed and extensive studies with a large number of patients have been conducted and published so far on the long-term effects of these devices, especially in older ages.
2. Objectives
We decided to compare the effect of closing ASDs by surgical methods or angiographic techniques in improving echocardiographic and clinical parameters in patients over 40 years of age.
3. Methods
The present study is a retrospective cohort study. The statistical population consisted of all ASD patients over 40 years of age who visited Shahid Rajai Hospital between 2016 and 2019 and underwent ASD device closure (180 patients) at this center. Given that the sample size depended on the number of archived cases, 180 patients were included in the ASD device closure group.
To collect data, in addition to obtaining demographic information such as age, gender, and clinical status, underlying diseases and symptoms, the patient's echocardiographic parameters were collected at 3, 6, and 12 months after the procedure, which were available in their file. To conduct this study, after obtaining ethics approval (IR.IUMS.FMD.REC.1400.284) and following legal procedures, necessary preparations were made. From 2016 to 2019, 213 patients with ASD, 180 of whom had undergone ASD device closure at Shaheed Rajai Cardiovascular Training and Treatment Center, were included.
Intrusion and less invasive methods have made great progress in terms of both equipment and quality of treatment and are currently considered one of the safest and least risky methods for treating congenital heart diseases, especially ASD. The principles of intrusion involve guiding a catheter to the heart using one of the peripheral veins, such as the femoral vein. These catheters are passed through the heart hole using a fluoroscopic guide that is performed by an angiography device. Then, using echocardiography guidance that is performed simultaneously with intrusion, the device is placed on both sides of the hole, and the heart hole is blocked. Over the next 6 months, this device is covered by a layer of cells from the inner layer of the heart and becomes part of the heart wall.
This procedure is performed under anesthesia, and during intrusion, anesthetic and sedative drugs are used to perform echocardiography and reduce the patient's stress. The intervention is performed in the angiography room, and the procedure takes about half an hour to an hour. In ASD other than the secundum ASD and in cases of secundum ASD that do not qualify for intrusion, the treatment of choice is open-heart surgery.
1. Inclusion criteria: All patients with secondary ASD over 40 years of age who visited Shaheed Rajai Hospital between 2016 and 2019, whose initial echocardiogram had been performed at this center, and who had undergone ASD device closure at this center were included.
2. Exclusion criteria: Patients with incomplete information were excluded from the study. This included cases of follow-up loss, patient withdrawal from participation, death, or the presence of other cardiac anomalies (e.g., crossing ASD).
To analyze the collected data and according to the hypotheses and research questions, descriptive statistics methods were used alongside chi-square and paired t-tests. Data analysis was performed using SPSS-21 statistical software. The presence of missing data was addressed by using different statistical methods: If the variable was quantitative, the empty cell was replaced with the mean; if the variable was on a Likert scale, the empty cell was replaced with the median; if the variable was nominal, the empty cell was replaced with the mode. This approach largely resolved the issue of missing data. Data analysis was performed using SPSS-21 statistical software.
4. Results
Overall, 180 patients underwent ASD device closure during the study period. The mean ± SD age of patients undergoing ASD device closure was 53.04 ± 10.72 years. The frequency distribution by gender showed that 70.6% of the patients were female. Comorbidities in patients undergoing ASD device closure included 58.3% with hypertension, 14.4% with diabetes, 21.1% with hyperlipidemia, 11.7% who were smokers, 2.4% with a history of stroke, and 0.6% with hereditary cardiovascular disease.
In cases of very large ASD sizes, there is a preference for surgery. In this study, the mean ± SD ASD size in the surgical group was 24.58 ± 5.68 mm. Based on the characteristics of ASD in patients who underwent ASD device closure, 7 patients had a patent foramen ovale (PFO), with 2 being large and 5 small. Six patients had a redundant interatrial septum (IAS), 4 had an aneurysmal IAS, 1 had a fenestrated IAS, and 3 patients had 2 ASDs. The smallest ASD size was 0.6 cm × 0.5 cm, and the largest was 2.75 cm × 2.50 cm (Table 1).
| Variables | ASD Device Closure |
|---|---|
| Features of ASD | |
| ASD size (mm) | 6.72 ± 20.33 |
| Antero inferior rim | 0.11 ± 1.29 |
| Poster inferior rim | 0.40 ± 1.28 |
| Antero superior rim | 0.06 ± 0.83 |
| Poster superior rim | 0.47 ± 1.60 |
| IVC rim | 0.63 ± 1.41 |
| SVC rim | 0.46 ± 1.23 |
| PFO | 7 (3.9) |
| Device specifications | |
| Amplatzer occluder | 13 (7.3) |
| Figulla occluder | 167 (92.7) |
| Device | 5.88 ± 23.84 |
Abbreviations: ASD, atrial septal defect; PFO, patent foramen ovale.
a Values are expressed as No. (%) or mean ± SD.
Based on the characteristics of the device used in patients who underwent ASD device closure, our results showed that devices were used to close ASD in about 93% of cases. The smallest device size was 9 mm, and the largest was 36 mm (Table 1).
Based on clinical symptoms before the procedure, 3 to 6 months after the procedure, and 12 months after the procedure in patients who underwent ASD device closure, the results showed that symptoms significantly decreased during the 3 to 6 months after the procedure and continued to improve at the 12-month follow-up (Table 2).
| Variables | Before the Procedure | 3 to 6 mo After the Procedure | 12 mo After the Procedure | P-Value |
|---|---|---|---|---|
| Clinical signs | 0.040 | |||
| Asymptomatic | 4 (2.2) | 22 (14.4) | 46 (28.9) | |
| Palpitation | 9 (5.0) | 2 (1.1) | 2 (1.1) | |
| Atypical CP | 6 (3.3) | 5 (2.8) | 6 (3.3) | |
| DOE | ||||
| Class 1 | 18 (10.0) | 4 (2.2) | 41 (22.8) | |
| Class 2 | 117 (65.0) | 52 (28.9) | 43 (23.9) | |
| Class 3 | 2 (1.1) | 0 (0) | 0 (0) | |
| LVEF (mean ± SD) | 4.80 ± 50.28 | 3.85 ± 52.81 | 3.66 ± 55.76 | 0.001 |
| LV diastolic dysfunction | 0.001 | |||
| None | 94 (52.2) | 115 (63.9) | 128 (71.1) | |
| Mild | 82 (45.6) | 64 (35.6) | 51 (28.3) | |
| Moderate | 4 (2.3) | 1 (0.6) | 1 (0.6) | |
| Severe | 0 (0) | 0 (0) | 0 (0) | |
| RV function | 0.001 | |||
| Normal | 33 (18.2) | 67 (37.0) | 81 (45.0) | |
| Preserved | 9 (5.0) | 6 (3.3) | 22 (12.9) | |
| Mild dysfunction | 85 (47.0) | 78 (44.0) | 62 (36.3) |
Abbreviations: LVEF, left ventricular ejection fraction; LV, left ventricular; RV, right ventricular.
a Values are expressed as No. (%) unless indicated.
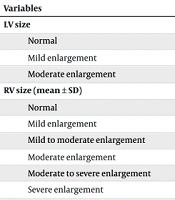
The frequency distribution based on left ventricular (LV) size before the procedure, 3 to 6 months after the procedure, and 12 months after the procedure in patients who underwent ASD device closure is detailed in Table 3. The mean and standard deviation of RV size before the procedure, 3 to 6 months after the procedure, and 12 months after the procedure showed that the mean RV size significantly decreased during the 3 to 6 months after the procedure and continued to decrease at the 12-month follow-up (Table 3).
| Variables | Before the Procedure | 3 to 6 mo After the Procedure | 12 mo After the Procedure | P-Value |
|---|---|---|---|---|
| LV size | 0.139 | |||
| Normal | 167 (92.7) | 176 (97.7) | 176 (97.7) | |
| Mild enlargement | 12 (6.7) | 3 (1.7) | 3 (1.7) | |
| Moderate enlargement | 1 (0.6) | 1 (0.6) | 1 (0.6) | |
| RV size (mean ± SD) | 6.16 ± 41.23 | 5.62 ± 37.18 | 4.50 ± 35.67 | 0.001 |
| Normal | 22 (12.2) | 86 (47.5) | 100 (55.6) | |
| Mild enlargement | 43 (23.8) | 49 (27.1) | 58 (33.9) | |
| Mild to moderate enlargement | 34 (18.8) | 26 (14.4) | 7 (4.1) | |
| Moderate enlargement | 44 (24.3) | 10 (5.5) | 5 (2.9) | 0.002 |
| Moderate to severe enlargement | 16 (8.8) | 6 (3.3) | 6 (3.3) | |
| Severe enlargement | 20 (11.0) | 3 (1.7) | 0 (0) | |
| LA size | 0.045 | |||
| Normal | 138 (76.2) | 156 (86.2) | 163 (90.6) | |
| Mild enlargement | 28 (15.5) | 16 (8.8) | 10 (5.6) | |
| Moderate enlargement | 5 (2.8) | 5 (2.8) | 5 (2.8) | |
| Severe enlargement | 9 (4.9) | 3 (1.7) | 2 (1.0) | |
| Normal | 88 (48.6) | 128 (70.7) | 137 (76.1) | |
| RA size | 0.006 | |||
| Mild enlargement | 47 (26.0) | 36 (19.9) | 31 (18.1) | |
| Moderate enlargement | 29 (16.0) | 10 (5.5) | 8 (4.7) | |
| Severe enlargement | 15 (8.4) | 5 (2.8) | 3 (1.7) |
Abbreviations: LV, left ventricular; RV, right ventricular; LA, left atrial; RA, right atrial.
a Values are expressed as No. (%) unless indicated.
Based on mitral regurgitation (MR) before the procedure, 3 to 6 months after the procedure, and 12 months after the procedure in patients who underwent ASD device closure, the results indicated that MR significantly improved during the 3 to 6 months after the procedure and continued to improve at the 12-month follow-up (Table 4).
| Variables | Before the Procedure | 3 to 6 mo After the Procedure | 12 mo After the Procedure | P-Value |
|---|---|---|---|---|
| MR | 0.048 | |||
| Normal | 7 (3.9) | 35 (19.3) | 40 (22.2) | |
| Mild | 145 (80.1) | 128 (71.1) | 122 (72.4) | |
| Mild to moderate | 16 (8.8) | 12 (6.6) | 14 (8.2) | |
| Moderate | 9 (5.0) | 5 (2.7) | 3 (1.7) | |
| Moderate to severe | 2 (1.0) | 0 (0) | 0 (0) | |
| Severe | 0 (0) | 7 (3.9) | 13 (7.2) | |
| TR | 0.040 | |||
| Mild | 66 (36.7) | 110 (60.8) | 106 (62.0) | |
| Mild to moderate | 33 (18.3) | 35 (19.3) | 43 (25.2) | |
| Moderate | 77 (38.8) | 27 (14.9) | 16 (9.4) | |
| Moderate to severe | 4 (2.2) | 0 (0) | 0 (0) | |
| Severe | 0 (0) | 0 (0) | 0 (0) | |
| AI | 0.812 | |||
| Normal | 130 (71.8) | 132 (72.9) | 116 (64.4) | |
| Mild | 49 (27.1) | 47 (26.0) | 64 (35.6) | |
| Moderate | 1 (1.1) | 1 (1.1) | 0 (0) |
Abbreviations: MR, mitral regurgitation; TR, tricuspid regurgitation; AI, aortic insufficiency.
a Values are expressed as No. (%).
Based on tricuspid regurgitation (TR) before the procedure, 3 to 6 months after the procedure, and 12 months after the procedure in patients who underwent ASD device closure, the results showed that TR significantly improved during the 3 to 6 months after the procedure and continued to improve at the 12-month follow-up (Table 4).
Based on aortic insufficiency (AI) before the procedure, 3 to 6 months after the procedure, and 12 months after the procedure in patients who underwent ASD device closure, the results are detailed in Table 4. The mean and standard deviation of systolic pulmonary artery pressure (PAP) before the procedure, 3 to 6 months after the procedure, and 12 months after the procedure in patients who underwent ASD device closure show that the mean systolic PAP decreased significantly during the 3 to 6 months after the procedure and continued to decrease at the 12-month follow-up. Based on systolic PAP before the procedure, 3 to 6 months after the procedure, and 12 months after the procedure in patients who underwent ASD device closure, the results indicated that the systolic PAP improved significantly during the 3 to 6 months after the procedure and continued to improve at the 12-month follow-up.
5. Discussion
The ASD accounts for approximately 6 - 10% of congenital heart diseases. In a review study conducted by van der Linde et al., the incidence of ASD was reported to be 1.64 cases per 1000 live births (7). Additionally, in another study conducted by Khan et al., about 10% of cases of congenital heart diseases in adults were ASDs (8). In Iran, studies have also investigated the incidence of this disorder. In a study conducted by Nikyar et al. in Gorgan, the incidence of ASD was reported to be 2.64 cases per 1000 live births (9).
Ostium secundum ASDs are often suitable for transcatheter procedures. However, percutaneous interventional procedures have limitations, including pulmonary venous occlusion, associated ASD anomalies, proximity of the ASD to the AV, coronary sinus, or venous drainage system, absence of a rim in a significant portion of the ASD, and lesions larger than 35 - 40 mm. The ASD device closure, when performed by an experienced practitioner, has very few complications, including device embolization, atrial arrhythmias, stroke, rupture, and thrombus formation in less than 1% of patients. In fact, ASD device closure is successful in improving functional class and exercise capacity.
In the past, open surgery was used to treat large ASDs. The first successful ASD device closure was performed by King et al. in 1976. This procedure was a great alternative to surgical treatment because it eliminated the need for thoracotomy, open-heart surgery with cardioplegia, and cardiopulmonary bypass. Other benefits of this method include reduced hospital stay, no post-surgical scars, reduced post-surgical pain, and a reduced incidence of dysrhythmias due to the absence of atrial scars (6-9).
Studies have shown that improvement in RV function and performance is achieved after ASD closure by transcatheter and surgical methods. However, complete information on the effect on LV function and other echocardiographic findings is not available, and LV and RV diastolic function remains uncertain. The effect of ASD closure on left atrial (LA) size is also unclear because a large LA can cause atrial dysrhythmias, atrial fibrillation (AF), stroke, and even death. Therefore, the aim of this study was to investigate the effect of ASD device closure on functional and echocardiographic findings with long-term follow-up.
The results of the present study showed that significant improvement in clinical symptoms was achieved during the 3 to 6 months and 12-month follow-up after the procedure in both ASD device closure and ASD surgical closure groups (9, 10). Most echocardiographic parameters, including left ventricular ejection fraction (LVEF), LV diastolic dysfunction, RV function, LV size, RV size, LA size, RA size, MR, TR, and systolic PAP, were significantly improved during the 3 to 6 months and 12-month follow-up in the ASD device closure group. However, in the ASD surgical closure group, only RV size and systolic PAP were significantly improved during the 3 to 6 months and 12-month follow-up.
These findings have also been reported in other studies investigating the therapeutic outcomes of ASD closure. For example, in a study conducted by Dhillon et al., all echocardiographic parameters increased equally in both the device and surgical groups (10). In a study conducted by Cheung et al., RV dysfunction was reported after ASD surgical closure, whereas RV function improved after ASD device closure (11).
Also, in a study by Hanseus et al., ASD device closure improved RV function (12). The results of the study by Hajizeinali et al. showed that left and RV function improved more in patients who underwent ASD device closure than in those who underwent surgery (13). The results of the study by Fang et al. indicated that the severity of TR decreased more after ASD device closure than after surgery (14). Salehiam et al. (as cited by Oechslin et al.) demonstrated in a study that ASD device closure improved RV and LV function and reduced LA size, which was associated with improved patient function (15). In a study conducted by Erwin Stroker et al., it was shown that ASD device closure improves the functional class, reduces RV size, reduces systolic pulmonary artery pressure (SPAP), increases LV size, and increases the E/A ratio (16).
In the studies by Salehian et al. (17), Teo et al. (18), and Poorzand et al. (19), a significant decrease in RV size, RA size, and PAP before and after surgery was noted. This finding can be justified considering the removal of the left-to-right shunt after surgery and, as a result, the reduction in blood flow to the right heart.
It is very important to follow up and care for these patients, and if care is provided appropriately, treatment will have a more favorable outcome (20-22). Proper follow-up can also increase the level of knowledge and attitude of patients, allowing them to participate actively in their own care (22-24).
5.1. Conclusions
In general, the device method leads to an improvement in clinical symptoms, although echocardiographic parameters improved more in the ASD device closure group. Therefore, in second-order ASD that meets the conditions for intrusion, the choice of device closure is preferable to open-heart surgery.