1. Background
The use of right ventricular pacemakers (RVPs) is a critical component in the management of advanced atrioventricular blocks (1), providing life-saving therapy and significantly enhancing the quality of life for millions of patients worldwide. Globally, more than 700,000 pacemakers are implanted annually (2), with a substantial proportion being RVP devices (1). While the clinical benefits of RVPs are well-established, there is increasing recognition of its significant complications, including pacing-induced cardiomyopathy (PiCM) (3), atrial fibrillation (AF) (4), and heart failure (HF) (5). The PiCM is a relatively common yet under-recognized complication of RVPs. Current estimates suggest that PiCM may develop in more than 10% of patients with RVP, depending on the population studied and the diagnostic criteria employed (6). Patients with PiCM typically present with new-onset HF symptoms after pacemaker implantation (1), leading to increased healthcare utilization and reduced quality of life. Moreover, the insidious progression of PiCM often goes undiagnosed due to the lack of unanimous diagnostic criteria, highlighting the need for early recognition and intervention, particularly before a significant reduction in left ventricular ejection fraction (LVEF) occurs. A major challenge in clinical practice and research is the lack of a universally accepted definition of PiCM, which limits comparability across studies and may delay diagnosis (7). The PiCM is usually defined by either a reduction of ≥ 10% in LVEF from baseline without any identifiable cause other than right ventricular pacing or an LVEF of ≤ 40% if the baseline LVEF was ≥ 50% (8). While the pathophysiology of PiCM is primarily attributed to pacing-induced ventricular dyssynchrony, often due to right ventricular apical pacing (9-11), its development is likely multifactorial. Contributing factors include patient characteristics and device-related variables such as pacing burden and lead position (12). Despite extensive research on PiCM, the predictors of this condition remain incompletely defined, underscoring the need for further investigation.
2. Objectives
The present study aims to assess the prevalence and determine independent risk factors of PiCM in an Iranian population with RVPs.
3. Methods
3.1. Study Protocol
This prospective observational study included patients who underwent right ventricle apical pacemaker implantation at Golestan and Imam Khomeini hospitals (tertiary centers in Ahvaz, Iran) between March 2021 to March 2023, using convenience sampling. The study followed the Declaration of Helsinki and was approved by Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.HGOLESTAN.REC.1401.064). After a thorough explanation of the study protocol, informed consent was obtained from participants. Demographic and medical history data were collected from records. All participants underwent transthoracic echocardiography by a board-certified echocardiologist before implantation and at six months post-implantation.
3.2. Echocardiographic Study
The LVEF was assessed using a Vivid E9 ultrasound machine (GE Vingmed Ultrasound, Horten, Norway) applying the biplane Simpson’s method of disc summation. This method was used in accordance with the guidelines of the American Society of Echocardiography and the European Association of Cardiovascular Imaging (13).
3.3. Definition
The PiCM was defined using the following criteria: (1) An LVEF ≤ 40% in patients with a baseline LVEF of ≥ 50% prior to pacemaker implantation; or (2) a reduction in LVEF of ≥ 10% in patients with a baseline LVEF < 50% prior to implantation. The chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate of less than 60 mL/min/1.73 m2 and/or an albumin-to-creatinine ratio of ≥ 30 mg/g, calculated using the CKD-EPI equation (14-16). Diabetes mellitus (DM) was defined by a history of glucose-lowering medication use or lab results meeting American Diabetes Association diagnostic criteria (17). Hyperlipidemia (HLP) was defined by current use of lipid-lowering therapy or meeting diagnostic criteria by the National Cholesterol Education Program ATP III and ACC/AHA guidelines (18).
3.4. Exclusion Criteria
Patients were excluded if they developed new-onset HF or cardiomyopathy due to causes other than PiCM, such as myocardial infarction, uncontrolled hypertension (HTN), and acute valvular diseases. Those unable to attend the six-month follow-up echocardiography were also excluded from the final analysis.
3.5. Statistical Analysis
Continuous data were expressed as means and standard deviations, while categorical data were presented as frequencies and percentages. The Kolmogorov-Smirnov test was employed to assess data normality. Group comparisons used independent samples t-tests or Mann-Whitney U tests (for non-normal data), and chi-square tests for categorical variables. Regression analysis evaluated associations with PiCM. Analyses were performed using SPSS version 27.0 (IBM Corp., Armonk, NY), with P < 0.05 considered statistically significant.
4. Results
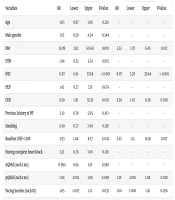
Initially, 110 patients were enrolled in the study. One patient was excluded due to myocardial infarction during follow-up, and four patients were excluded due to missed follow-up, leaving 105 for final analysis. The mean age of the participants was 71.2 ± 8.96 years, with a female predominance (n = 63, 60.0%). Primarily, 59 patients (56.2%) had complete heart block, 40 had high-grade atrioventricular block (38.1%), and the remaining six patients had either low response AF or sick sinus syndrome. The most prevalent comorbidity among the study population was HTN (n = 75, 71.4%), followed by DM (n = 49, 46.7%) and HF (n = 32, 30.5%). At six months, 18 patients (17.1%) developed PiCM (Table 1). No significant differences in demographics or baseline LVEF were found between included and excluded patients (Appendix 1 in Supplementary File).
| Variables | Total Population (N = 105) | PiCM (n = 18) | Non-PiCM (n = 87) | P-Value |
|---|---|---|---|---|
| Age (y) | 71.25 ± 8.96 | 73.05 ± 9.40 | 70.39 ± 9.07 | 0.262 |
| Sex | 0.341 | |||
| Male | 42 (40.0) | 9 (50.0) | 33 (38.0) | |
| Female | 63 (60.0) | 9 (50.0) | 54 (62.0) | |
| HTN | 75 (71.4) | 13 (72.2) | 62 (71.2) | 0.935 |
| DM | 49 (46.7) | 16 (88.8) | 33 (37.9) | < 0.001 |
| Previous history of HF | 32 (30.5) | 8 (44.4) | 24 (27.5) | 0.157 |
| IHD | 28 (26.7) | 12 (66.6) | 16 (18.4) | < 0.001 |
| HLP | 10 (9.5) | 2 (11.1) | 8 (9.2) | 0.672 |
| CKD | 5 (4.7) | 3 (16.7) | 2 (2.3) | 0.009 |
| Smoking | 21 (20.0) | 6 (33.3) | 15 (17.2) | 0.120 |
Abbreviations: PiCM, pacing-induced cardiomyopathy; HTN, hypertension; DM, diabetes mellitus; HF, heart failure; IHD, ischemic heart disease; HLP, hyperlipidemia; CKD, chronic kidney disease.
a Values are expressed as No. (%) or mean ± SD.
4.1. Comparison Between Pacing-Induced Cardiomyopathy and Non-pacing-Induced Cardiomyopathy Patients
In this study, PiCM patients had significantly higher rates of DM (P < 0.001), IHD (P < 0.001), and CKD (P = 0.009). They also exhibited lower LVEF after six months (P = 0.021), longer paced QRS duration (pQRSd) (P = 0.042), and higher pacing burden (P = 0.032). No significant differences were observed between the PiCM and non-PiCM groups regarding age, gender, HTN, HF, HLP, smoking, type of AV block, or native QRS duration (nQRSd) (All P > 0.05) (Tables 1 and 2).
| Variables | Total Population (N = 105) | PiCM (n = 18) | Non-PiCM (n = 87) | P-Value |
|---|---|---|---|---|
| Primary rhythm | 0.094 | |||
| Complete heart block | 59 (56.2) | 13 (72.2) | 46 (52.9) | |
| High grade AV block | 40 (38.1) | 3 (16.7) | 37 (43.5) | |
| Low-response AF/sick sinus syndrome | 6 (5.7) | 2 (11.1) | 4 (4.6) | |
| Baseline LVEF (%) | 48.27 ± 8.65 | 46.38 ± 9.20 | 48.67 ± 8.54 | 0.309 |
| LVEF at 6-months follow-up (%) | 49.46 ± 11.92 | 43.61 ± 17.72 | 50.68 ± 10.06 | 0.021 |
| nQRSd (ms) | 106.31 ± 11.76 | 104.09 ± 17.14 | 106.78 ± 10.65 | 0.387 |
| pQRSd (ms) | 145.33 ± 11.62 | 150.43 ± 14.64 | 144.28 ± 11.0 | 0.042 |
| Pacing burden (%) | 72.80 ± 11.62 | 78.22 ± 11.59 | 71.68 ± 11.68 | 0.032 |
Abbreviations: PiCM, pacing-induced cardiomyopathy; AV, atrioventricular; AF, atrial fibrillation; LVEF, left ventricular ejection fraction; nQRSd, native QRS duration; pQRSd, paced QRS duration.
a Values are expressed as No. (%) or mean ± SD.
4.2. Predictors of Pacing-Induced Cardiomyopathy
Univariable regression revealed significant associations between PiCM and DM (OR = 13.09; 95% CI 2.82 - 60.60; P = 0.001), IHD (OR = 11.87; 95% CI 6.16 - 57.68; P < 0.001), CKD (OR = 8.50; 95% CI 1.30 - 55.23; P = 0.025), baseline LVEF < 50% (OR = 2.93; 95% CI 1.04 - 8.27; P = 0.042), pQRSd (OR = 1.04; 95% CI 1.002 - 1.09; P = 0.049), and pacing burden (OR = 1.06; 95% CI 1.005 - 1.12; P = 0.032) (Table 3). In multivariable analysis, independent predictors of PiCM included DM (OR = 3.53; 95% CI 1.78 - 6.45; P = 0.012), IHD (OR = 8.07; 95% CI 5.20 - 29.64; P < 0.001), and CKD (OR = 5.29; 95% CI 1.47 - 11.29; P = 0.028). Baseline LVEF < 50% increased PiCM risk 5.67-fold (95% CI 1.15 - 16.38; P = 0.017). Each one millisecond increase in pQRSd was associated with a 1% increase in PiCM odds (95% CI 1.001 - 1.08; P = 0.038) and each percentage increase in pacing burden raised the risk by 6% (95% CI 1.008 - 1.26; P = 0.036) (Table 3). Receiver operator curve analysis demonstrated that pacing burden > 65% can predict PiCM (AUC = 0.651; 95% CI 0.552 - 0.742; P = 0.033) with 94.4% sensitivity and 28.7% specificity.
| Variables | Univariable Regression Analysis | Multivariable Regression Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-Value | OR | 95% CI | P-Value | |||
| Lower | Upper | Lower | Upper | |||||
| Age | 1.03 | 0.97 | 1.09 | 0.261 | - | - | - | - |
| Male gender | 1.63 | 0.59 | 4.54 | 0.344 | - | - | - | - |
| DM | 13.09 | 2.82 | 60.60 | 0.001 | 3.53 | 1.78 | 6.45 | 0.012 |
| HTN | 1.04 | 0.33 | 3.24 | 0.935 | - | - | - | - |
| IHD | 11.87 | 6.16 | 57.68 | < 0.001 | 8.07 | 5.20 | 29.64 | < 0.001 |
| HLP | 1.42 | 0.27 | 7.51 | 0.674 | - | - | - | - |
| CKD | 8.50 | 1.30 | 55.23 | 0.025 | 5.29 | 1.47 | 11.29 | 0.028 |
| Previous history of HF | 2.10 | 0.74 | 5.95 | 0.163 | - | - | - | - |
| Smoking | 2.40 | 0.77 | 7.40 | 0.128 | - | - | - | - |
| Baseline LVEF < 50% | 2.93 | 1.04 | 8.27 | 0.042 | 5.67 | 1.15 | 16.38 | 0.017 |
| Having complete heart block | 2.31 | 0.76 | 7.06 | 0.139 | - | - | - | - |
| nQRSd (each 1 ms) | 0.994 | 0.96 | 1.01 | 0.580 | - | - | - | - |
| pQRSd (each 1 ms) | 1.04 | 1.002 | 1.09 | 0.049 | 1.01 | 1.001 | 1.08 | 0.038 |
| Pacing burden (each 1%) | 1.05 | 1.005 | 1.12 | 0.032 | 1.06 | 1.008 | 1.26 | 0.036 |
Abbreviations: OR, odds ratio; CI, confidence interval; DM, diabetes mellitus; HTN, hypertension; IHD, ischemic heart disease; HLP, hyperlipidemia; CKD, chronic kidney disease; HF, heart failure; LVEF, left ventricular ejection fraction; nQRSd, native QRS duration; pQRSd, paced QRS duration; ms, Millisecond.
5. Discussion
In this study, 17.1% of patients who underwent RVP developed PiCM within six months. These patients had significantly higher rates of DM, IHD, and CKD, and greater pacing burden. Independent risk factors included baseline LVEF < 50%, and histories of CKD, IHD, and DM. While RVP remains a standard treatment for atrioventricular block, especially with an aging population, it poses risks, including PiCM. Diagnosis is primarily based on LVEF assessment via conventional methods, which are widely accessible and reasonably reproducible (19). Previous studies report PiCM prevalence ranging from 6% to 25%, depending on definitions and follow-up durations (20-25). Our observed rate of 17.1% in apical RVP patients aligns with these findings. While no significant differences were found between the PiCM and non-PiCM groups in our population, consistent with some studies (26-28), other research has identified baseline LVEF as a distinguishing factor (22-24, 29). In our study, patients with baseline LVEF < 50% showed higher odds of developing PiCM. Similarly, previous studies reported that higher baseline LVEF was associated with a lower PiCM risk, confirming LVEF as an independent predictor (23-25, 29). Variations in baseline LVEF findings could be attributed to methodological differences. Some studies included only patients with LVEF ≥ 50% to exclude those with pre-existing HF, although HF with reduced ejection fraction is typically defined as LVEF ≤ 40%, according to the AHA/ACC/HFSA guidelines. The absence of a standardized PiCM definition further complicates comparisons. While some define PiCM as a ≥ 10% LVEF reduction from a baseline ≥ 50%, others include patients with baseline LVEF < 50% if a further 5 - 10% decline occurs (8). A unified definition could improve early diagnosis and identification of high-risk patients.
In our study, RV pacing burden was significantly higher in patients who developed PiCM, with each percentage increase linked to a 6% rise in risk of PiCM. This aligns with prior studies, including a meta-analysis by Somma et al., which found a 2% increase in PiCM odds per 1% increase in pacing burden (12). While high RV pacing is a recognized risk factor, the optimal threshold remains debated. We identified 65% as a predictive cut-off, compared to 60% reported by Bansal et al. (28), 40% in other studies (8, 30), and even 20% by Kiehl et al. (29). Our higher threshold may reflect the generally elevated pacing burden in our cohort. Although an AUC of 0.651 reflects modest discrimination, this threshold may still aid broader clinical risk assessment. Pacing burden alone is not definitive and should be interpreted alongside baseline LVEF, comorbidities, and QRS duration. Including it in a multifactorial model may improve early identification of at-risk patients. Larger prospective studies are needed to refine pacing burden thresholds for PiCM risk stratification.
The effects of native and pQRSd on the development of PiCM remain controversial, with heterogeneous findings reported in the literature. Khurshid et al. identified nQRSd as a significant predictor of PiCM, while pQRSd was not associated with PiCM in their analysis (31). However, in a subsequent study by the same authors in 2016, no association was found between nQRSd and PiCM, whereas pQRSd was significantly related to PiCM development (32). Similarly, Cho et al. demonstrated that pQRSd was an independent risk factor for PiCM, with no significant association observed for nQRSd (22). A 15-year study by Kim et al. on the South Korean population further supported pQRSd as an independent predictor of PiCM (26). Similarly, we also observed that pQRSd was independently associated with PiCM, while nQRSd did not show any significant association with PiCM development. In contrast with the above-mentioned studies and our findings, a recent meta-analysis by Somma et al. reported that both nQRSd and pQRSd were potential risk factors for PiCM (12). Current evidence suggests that increased pQRSd plays a critical role in PiCM risk, though the findings for nQRSd remain inconclusive. Larger studies are needed to clarify the role of nQRSd, as it has been unexplored in existing literature; for instance, Somma et al.’s meta-analysis included ten studies on pQRSd but only three on nQRSd.
In addition to pacing burden and pQRSd, comorbidities such as DM, IHD, and CKD have been proposed earlier as risk factors of PiCM (12, 22, 33, 34). Other studies have suggested atrioventricular blocks, AF, and prolonged QRS duration prior to pacing with increased PiCM risk (5, 12, 35, 36). Our findings align with these observations, as patients with a history of DM, IHD, or CKD demonstrated significantly increased risk of PiCM. However, some studies have not reported such associations (26-28), likely due to heterogeneity among study populations, varying PiCM definitions, and differences in follow-up durations. Currently, our ability to discriminate patients with high risk of PiCM from others is limited, and these inconsistencies highlight the need for more research to clarify the role of comorbidities in PiCM risk and to improve patient monitoring and outcomes.
5.1. Conclusions
The prevalence of PiCM in the Iranian population is consistent with global data. Diabetes mellitus, IHD, CKD, baseline LVEF < 50%, prolonged pQRSd, and increased pacing burden were identified as independent risk factors in patients with right ventricular apical pacemakers. These findings highlight the need for early detection and close monitoring of high-risk patients to mitigate PiCM-related complications. Identifying at-risk individuals may also prompt consideration of alternative pacing strategies, such as cardiac resynchronization therapy or left bundle branch area pacing, instead of right ventricular apical pacing for atrioventricular block management.
5.2. Study Limitations
To our knowledge, this is the first study to assess PiCM risk factors in an Iranian population. However, several limitations must be acknowledged. The relatively small sample size and recruitment from a single region may limit generalizability. A limited sample may also contribute to overfitting in regression models. The six-month follow-up might have been too short to capture all outcomes; longer follow-up could provide more definitive insights. Selection bias may have occurred due to the exclusion of patients lost to follow-up. Although baseline characteristics between included and excluded patients showed no significant differences, this could still affect external validity. The sample size also prevented formal sensitivity analyses to test the robustness of our findings. Lastly, we did not assess reproducibility (e.g., interobserver variability in echocardiographic measurements), which may limit evaluation consistency.