1. Background
Cardiovascular complications in COVID-19 are highly significant. Patients with pre-existing heart conditions such as coronary artery disease, heart failure, hypertension, and diabetes tend to have worse outcomes with COVID-19 (1).
The presence of two cardiac comorbidities increased the risk of intensive care unit (ICU) admission, mechanical ventilation, or mortality by 2.59 times (2). Earlier studies had ascertained that cardiovascular disease (CVD) as an underlying comorbidity might enhance the risk of mortality in COVID-19 patients (3, 4). Acute myocardial injury in COVID-19 patients raises morbidity and mortality, leading to more complications such as acute respiratory distress syndrome (ARDS), arrhythmias, coagulopathy, kidney injury, and acute coronary syndromes caused by heightened coagulation and inflammation (5). Patients with ST-elevation myocardial infarction (STEMI) and COVID-19 had a 21% higher death risk, showing a bidirectional link between the conditions (6). COVID-19 increases thrombotic complications due to interactions with platelets, microvascular injury, cytokine release, and endothelial dysfunction (7). COVID-19 patients showed a 17% incidence of venous thromboembolism (VTE), including deep vein thrombosis (DVT), which was more common in hospitalized than non-hospitalized patients (8). The prevalence of DVT was around 12.1%, and pulmonary thromboembolism (PTE) was approximately 7.1% in these patients (9). In addition, COVID-19 patients experienced catheter-related thrombosis, filter thrombosis during renal therapy, and portal and mesenteric vein thrombosis (10).
Based on past research, the relationship between COVID-19 and CVD remains unclear and appears to be a bidirectional relationship. Pre-existing CVD is common in patients hospitalized with COVID-19. In addition, COVID-19 has been associated with cardiovascular complications, such as myocardial infarction or injury, myocarditis, heart failure, arrhythmia, and stroke, even in patients without a history of CVD. However, more studies are needed to address this paradox (11, 12).
2. Objectives
This study aimed to investigate the cardiovascular links to ICU admission and mortality in COVID-19 patients.
3. Methods
In a retrospective and applied study, 216 COVID-19 patients selected from Shahid Faghihi Hospital were examined. Patients were enrolled in this cohort based on a positive PCR test for SARS-CoV-2 and an age range of 18 to 75 years. This study aimed to determine factors that affect the likelihood of these patients being transferred to intensive care, dying, or being discharged from the hospital. Clinical data of the patients were collected from the hospital information system to determine the relationship between demographic factors, cardiovascular outcomes, and clinical indicators with determining intensive care status, death, and discharge using statistical methods. In this analysis, the role of factors such as age, gender, and cardiovascular indicators, including cardiac evaluation results and the presence or absence of positive troponin, was examined alongside ECG findings to provide quantitative predictions of disease severity and risk of cardiovascular complications.
Inclusion criteria included patients with a positive PCR test. Patients whose test results were not known and those not in the adult age range were excluded from the study. Clinical data were based on the hospital information system, and a cardiologist reviewed the clinical data to remove erroneous data. The study protocol was developed by consensus of experts and used the steps of managing missing data through multiple imputation to ensure the validity and neutrality of the data.
The collected data were analyzed using IBM SPSS version 26. Qualitative variables were reported as numerical-relative (number and percentage), and quantitative variables were reported as descriptive with mean and standard deviation to provide a clear picture of the sample distribution. Patients were grouped according to three main criteria: Hospitalization department and clinical outcomes.
The statistical tests used in this study included chi-square for binary qualitative data, t-tests for comparing the means of two groups, and the Mann-Whitney test for nonparametric comparisons between groups. For cardiovascular outcome analysis, a logistic regression model with the enter method was used to predict the association between clinical indicators and cardiovascular events while controlling for confounding factors such as age and underlying diseases to improve model validity and inferential power. This approach allows for the simultaneous influence of multiple factors on clinical outcomes and reduces bias due to overlapping variables.
4. Results
This study involved 216 patients, with 41.2% being male. Outcomes included 14.8% deaths and 23.1% ICU admissions. The mean ± SD age was 61.6 13.9 years.
4.1. Intensive Care Unit Admission
Among 166 ward patients, 39.8% were male, while of the 50 ICU patients, 46.0% were male. There was no significant gender difference in ICU admission (P = 0.432). The mean age of ICU patients was 65.94 years, while the mean age of ward patients was 60.25 years (Table 1).
| Variables | Regular Ward (N = 166) | ICU Admission (N = 50) | P-Value |
|---|---|---|---|
| Gender | 0.432 | ||
| Man | 66 (39.8) | 23 (46.0) | |
| Woman | 100 (60.2) | 27 (54.0) | |
| Age | 60.25 ± 13.985 | 65.94 ± 12.643 | 0.011 |
Abbreviation: ICU, intensive care unit.
a Values are expressed as mean ± SD or No. (%).
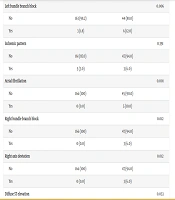
This study compared ECG findings between ICU and ward patients. Only 6% of ICU patients had a normal ECG, compared to 54.8% in the wards (P < 0.001). Common ICU abnormalities included sinus tachycardia, left bundle branch block, atrial fibrillation, right bundle branch block, and rightward axis deviation, all with significant P-values (Table 2).
| ECG Findings | Regular Ward (N = 166) | ICU Admission (N = 50) | P-Value |
|---|---|---|---|
| Normal | < 0.001 | ||
| No | 75 (45.2) | 47 (94.0) | |
| Yes | 91 (54.8) | 3 (6.0) | |
| Sinus tachycardia | < 0.001 | ||
| No | 143 (86.1) | 32 (64.0) | |
| Yes | 23 (13.9) | 18 (36.0) | |
| Nonspecific ST-T changes | 0.175 | ||
| No | 140 (84.3) | 38 (76.0) | |
| Yes | 26 (15.7) | 12 (24.0) | |
| Sinus bradycardia | 0.073 | ||
| No | 154 (92.8) | 50 (100) | |
| Yes | 12 (7.2) | 0 (0.0) | |
| Left bundle branch block | 0.006 | ||
| No | 163 (98.2) | 44 (88.0) | |
| Yes | 3 (1.8) | 6 (12.0) | |
| Ischemic pattern | 0.391 | ||
| No | 161 (97.0) | 47 (94.0) | |
| Yes | 5 (3.0) | 3 (6.0) | |
| Atrial fibrillation | 0.001 | ||
| No | 166 (100) | 45 (90.0) | |
| Yes | 0 (0.0) | 5 (10.0) | |
| Right bundle branch block | 0.012 | ||
| No | 166 (100) | 47 (94.0) | |
| Yes | 0 (0.0) | 3 (6.0) | |
| Right axis deviation | 0.012 | ||
| No | 166 (100) | 47 (94.0) | |
| Yes | 0 (0.0) | 3 (6.0) | |
| Diffuse ST elevation | 0.053 | ||
| No | 166 (100) | 48 (96.0) | |
| Yes | 0 (0.0) | 2 (4.0) |
Abbreviation: ICU, intensive care unit.
a Values are expressed as No. (%).
The study found that pericardial effusion was more common in ICU patients (18%) compared to ward patients (3%, P < 0.001). Similarly, pleural effusion was more prevalent in ICU patients (30%) versus ward patients (9.6%, P < 0.001). Bilateral pleural effusion was significantly higher in ICU patients (24%) than in ward patients (4.8%, P < 0.001), while no significant difference was observed in unilateral effusion (P = 0.719, Table 3).
| Fluid Accumulations | Regular Ward (N = 166) | ICU Admission (N = 50) | P-Value |
|---|---|---|---|
| Pericardial effusion | 0.001 | ||
| No | 161 (97.0) | 41 (82.0) | |
| Yes | 5 (3.0) | 9 (18.0) | |
| Pleural effusion overall | < 0.001 | ||
| No | 150 (90.4) | 35 (70.0) | |
| Yes | 16 (9.6) | 15 (30.0) | |
| Unilateral pleural effusion | 0.719 | ||
| No | 158 (95.2) | 47 (94.0) | |
| Yes | 8 (4.8) | 3 (6.0) | |
| Bilateral pleural effusion | < 0.001 | ||
| No | 158 (95.2) | 38 (76.0) | |
| Yes | 8 (4.8) | 12 (24.0) |
Abbreviation: ICU, intensive care unit.
a Values are expressed as No. (%).
This study examined vascular complications in COVID-19 patients related to ICU hospitalization. Stroke occurred in 3% of ward patients and none in ICU patients (P = 0.592), showing no significant difference. The DVT was absent in ward patients but present in 6% of ICU patients (P = 0.012). The PTE affected 10.2% of ward patients and 18% of ICU patients (P = 0.139), which was not significantly different. However, massive pulmonary embolism was more common in ICU patients (6% vs. 0%; P = 0.012, Table 4).
| Vascular Complications | Regular Wards (N = 166) | ICU Admission (N = 50) | P-Value |
|---|---|---|---|
| Stroke | 0.592 | ||
| No | 161 (97.0) | 50 (100) | |
| Yes | 5 (3.0) | 0 (0.0) | |
| DVT | 0.012 | ||
| No | 166 (100) | 47 (94.0) | |
| Yes | 0 (0.0) | 3 (6.0) | |
| PTE | 0.139 | ||
| No | 149 (89.8) | 41 (82.0) | |
| Yes | 17 (10.2) | 9 (18.0) | |
| Pulmonary sub-segmental thromboembolism | 0.216 | ||
| No | 155 (93.4) | 44 (88.0) | |
| Yes | 11 (6.6) | 6 (12.0) | |
| Pulmonary segmental thromboembolism | 0.34 | ||
| No | 160 (96.4) | 50 (100) | |
| Yes | 6 (3.6) | 0 (0.0) | |
| Massive PTE | 0.012 | ||
| No | 166 (100) | 47 (94.0) | |
| Yes | 0 (0.0) | 3 (6.0) |
Abbreviations: ICU, intensive care unit; DVT, deep vein thrombosis; PTE, pulmonary thromboembolism.
a Values are expressed as No. (%).
The study's regression analysis showed that older age, positive troponin, sinus tachycardia, left bundle branch block, pericardial effusion, and pleural effusions significantly increased the likelihood of ICU admission, with troponin and age having the strongest associations (Table 5).
| Variables | OR | P-Value |
|---|---|---|
| Age | 1.032 | 0.012 |
| Troponin | 4.655 | < 0.001 |
| D-dimer | 1.000 | < 0.001 |
| Normal ECG | 0.053 | < 0.001 |
| Sinus tachycardia | 3.497 | < 0.001 |
| Left bundle branch block | 7.409 | 0.006 |
| Pericardial effusion | 7.068 | 0.001 |
| Overall pleural effusion | 2.435 | < 0.001 |
| Bilateral pleural effusion | 6.237 | < 0.001 |
Abbreviation: OR, odds ratio.
4.2. Mortality
In this study, among 184 recovered patients, 40.8% were male, while among the 32 deceased patients, 43.8% were male. There was no significant association between gender and mortality (P = 0.751). The results revealed that deceased patients were significantly older (P < 0.001, Table 6).
| Variables | Outcomes | P-Value | |
|---|---|---|---|
| Recovery (N = 184) | Death (N = 32) | ||
| Gender | 0.751 | ||
| Man | 75 (40.8) | 14 (43.8) | |
| Woman | 109 (59.2) | 18 (56.3) | |
| Age | 60.08 ± 13.81 | 70.13 ± 10.86 | < 0.001 |
| Troponin | < 0.001 | ||
| Negative | 76 (56.7) | 3 (9.4) | |
| Borderline | 46 (34.3) | 7 (53.1) | |
| Positive | 12 (9.0) | 12(37.5) | |
| D-dimer | 1559.8 ± 1886.1 | 4404.8 ± 4171.8 | < 0.001 |
a Values are expressed as mean ± SD or No. (%).
Troponin levels were categorized as negative, borderline, or positive in 166 patients, with 47.5%, 38.0%, and 14.5%, respectively. D-dimer was measured in 209 patients, averaging around 2580, with deceased patients showing significantly higher levels (P < 0.001, Table 6).
This study found no normal ECGs in the 32 deceased patients, while 51.1% of the 184 recovered patients had normal ECGs (P < 0.001). Deceased patients more frequently showed non-specific ST-T changes, atrial fibrillation, right bundle branch block, rightward axis deviation, and diffuse ST elevation, all with significant P-values (Table 7).
| Findings | Recovery (N = 184) | Death (N = 32) | P-Value |
|---|---|---|---|
| ECG | |||
| Normal | < 0.001 | ||
| No | 90 (48.9) | 32 (100) | |
| Yes | 94 (51.1) | 0 (0.0) | |
| Sinus tachycardia | 0.971 | ||
| No | 149 (81.0) | 26 (81.3) | |
| Yes | 35 (19.0) | 6 (18.8) | |
| Nonspecific ST-T changes | 0.001 | ||
| No | 158 (85.9) | 20 (62.5) | |
| Yes | 26 (14.1) | 12 (37.5) | |
| Sinus bradycardia | 0.221 | ||
| No | 172 (93.5) | 32 (100) | |
| Yes | 12 (6.5) | 0 (0.0) | |
| Left bundle branch block | 0.133 | ||
| No | 178 (96.7) | 29 (90.6) | |
| Yes | 6 (3.3) | 3 (9.4) | |
| Ischemic pattern | 0.098 | ||
| No | 179 (97.3) | 29 (90.6) | |
| Yes | 5 (2.7) | 3 (9.4) | |
| Atrial fibrillation | < 0.001 | ||
| No | 184 (100) | 27 (84.4) | |
| Yes | 0 (0.0) | 5 (15.6) | |
| Right bundle branch block | 0.003 | ||
| No | 184 (100) | 29 (90.6) | |
| Yes | 0 (0.0) | 3 (9.4) | |
| Right axis deviation | 0.003 | ||
| No | 184 (100) | 29 (90.6) | |
| Yes | 0 (0.0) | 3 (9.4) | |
| Diffuse ST elevation | 0.021 | ||
| No | 184 (100) | 30 (93.8) | |
| Yes | 0 (0.0) | 2 (6.3) | |
| Echocardiographic | |||
| Normal | 0.183 | ||
| No | 17 (50.0) | 9 (75.0) | |
| Yes | 17 (50.0) | 3 (25.0) | |
| LVD | 0.091 | ||
| No | 20 (58.8) | 3 (25.0) | |
| Yes | 14 (41.2) | 9 (75.0) | |
| RVD | 0.005 | ||
| No | 31 (91.2) | 6 (50.0) | |
| Yes | 3 (8.8) | 6 (50.0) |
a Values are expressed as No. (%).
The study examined echocardiographic findings and outcomes. Half of the recovered patients had normal echocardiograms, compared to 25% of deceased patients (P = 0.183), which was not statistically significant. Reduced left ventricular function was seen in 41.2% of recovered and 75% of deceased patients (P = 0.091), also not significant. However, deceased patients were significantly more likely to have reduced right ventricular function (50% vs. 8.8%; P = 0.005, Table 7).
Deceased patients were more likely to have pericardial effusion (18.8% vs. 4.3%, P = 0.008) and pleural effusion (37.5% vs. 10.3%, P < 0.001). Regarding unilateral effusion, there was no significant difference between groups (P = 0.375). However, bilateral pleural effusion was significantly higher in deceased patients (37.5% vs. 4.3%, P < 0.001, Table 8).
| Fluid Accumulations | Recovery (N = 184) | Death (N = 32) | P-Value |
|---|---|---|---|
| Pericardial effusion | 0.002 | ||
| No | 176 (95.7) | 26 (81.3) | |
| Yes | 8 (4.3) | 6 (18.8) | |
| Pleural effusion overall | < 0.001 | ||
| No | 165 (89.7) | 20 (62.5) | |
| Yes | 19 (10.3) | 12 (37.5) | |
| Unilateral pleural effusion | 0.375 | ||
| No | 173 (94.0) | 32 (100) | |
| Yes | 11 (6.0) | 0 (0.0) | |
| Bilateral pleural effusion | < 0.001 | ||
| No | 176 (95.7) | 20 (62.5) | |
| Yes | 8 (4.3) | 12 (37.5) |
a Values are expressed as No. (%).
This study found no significant differences between recovered and deceased COVID-19 patients in the occurrence of stroke, DVT, or PTE overall, with P-values > 0.99 and 0.206. However, sub-segmental pulmonary embolism was significantly more common in deceased patients (18.8%) compared to recovered patients (6.0%, P = 0.013, Table 9).
| Vascular Complications | Recovery (N = 184) | Death (N = 32) | P-Value |
|---|---|---|---|
| Stroke | > 0.999 | ||
| No | 179 (97.3) | 32 (100) | |
| Yes | 5 (2.7) | 0 (0.0) | |
| DVT | > 0.999 | ||
| No | 181 (98.4) | 32 (100) | |
| Yes | 3 (1.6) | 0 (0.0) | |
| PTE | 0.206 | ||
| No | 164 (89.1) | 26 (81.3) | |
| Yes | 20 (10.9) | 6 (18.8) | |
| Pulmonary sub-segmental thromboembolism | 0.013 | ||
| No | 173 (94.0) | 26 (81.3) | |
| Yes | 11 (6.0) | 6 (18.8) | |
| Pulmonary segmental thromboembolism | 0.595 | ||
| No | 178 (96.7) | 32 (100) | |
| Yes | 6 (3.3) | 0 (0.0) | |
| Massive PTE | 0.467 | ||
| No | 181 (98.4) | 32 (100) | |
| Yes | 3 (1.6) | 0 (0.0) |
Abbreviations: DVT, deep vein thrombosis; PTE, pulmonary thromboembolism.
a Values are expressed as No. (%).
5. Discussion
This study examined 216 COVID-19 patients, focusing on cardiovascular complications and comparing outcomes based on demographics, cardiovascular assessments, and vascular issues. Findings showed that ICU patients and those who died were older than recovered patients. Each additional year of age increased the odds of ICU hospitalization by 1.032 times and mortality by 1.062 times. These results are consistent with other research, such as Bonanad et al.'s meta-analysis, which also found higher mortality rates in older COVID-19 patients (13). Previous studies indicate that males had higher COVID-19 mortality rates than females, but the difference was not statistically significant (14). The study found mortality rates of 15.7% in males and 14.2% in females. Cohen et al. reported higher hospitalization rates for ages 30 - 69, while ICU admissions for those over 70 were lower than in younger groups (15). This difference in hospitalization rates may stem from an age-based approach to admission, especially when ICU beds were limited during COVID-19 in many areas (16).
In the study, 14.5% of hospitalized COVID-19 patients tested positive for troponin. This rate was higher among ICU and deceased patients compared to those in regular wards or who recovered. Al Abbasi et al. in Florida found 27.6% of hospitalized patients had positive troponin and were more likely to die (17). Similarly, in a study by Garcia de Guadiana-Romualdo et al., more than 12% of patients had positive troponin (18). The lower troponin threshold in this study may be due to a "borderline" troponin parameter in lab tests. However, troponin positivity in COVID-19 is likely linked to myocardial injury caused by respiratory issues and myocarditis-related hypoxia (19). In the studied group, only 56.5% had normal ECGs, with sinus tachycardia being the most common at 19%. This increased heart rate may result from physiological responses to infection and fever. Kaliyaperumal et al.’s study of over 300 COVID-19 patients in India found 2.9% had rhythm disorders like atrial fibrillation, and 16.9% showed ST-segment abnormalities. Cardiac rate issues were present in 36.5%, making it the most frequent ECG abnormality in that group (20). The significant rhythm disorders observed align with Indian research. In this study, sinus tachycardia, bundle branch blocks, atrial fibrillation, and right axis deviation were common during ICU hospitalization.
Echocardiography, performed only in patients with suspected heart failure, showed a 50% prevalence of reduced left ventricular function and 4.2% for right ventricular dysfunction, higher in ICU and deceased patients. Mele et al.’s study of 96 COVID-19 patients found subclinical left ventricular reduction in 90%, highlighting COVID-19’s severe impact on the heart (21). Pericardial effusion was seen in 6.5% of patients, and pleural effusion in 14.4%, both linked to higher ICU stays and mortality. The effect of pleural effusion was mainly noted with bilateral involvement, indicating more severe illness. Ghantous et al. found pericardial effusion in 14% of COVID-19 patients who all underwent echocardiography, with some showing no symptoms (22). The higher prevalence of pericardial effusion in Ghantous’ study likely results from all patients, including those without clinical signs, undergoing echocardiography. Vascular complications were also assessed, with cerebral infarction occurring in 2.3% of patients, DVT in 1.4%, and PTE in 14% of patients. Sub-segmental pulmonary embolisms were associated with higher mortality, possibly due to the initial undetected severity. Nannoni et al.’s 2021 meta-analysis estimated ischemic stroke prevalence at around 1.22%, similar to this study, with over 30% of stroke patients dying — much higher than mortality from COVID-19 alone (23). In this study, the stroke rate among critically ill patients was not significantly elevated, likely due to the inability to transfer some severely ill patients for CT scans. Additionally, Jig Ng’s 2021 meta-analysis reported that approximately 11.1% of COVID-19 patients on prophylactic anticoagulation experienced PTE (24). This is roughly consistent with the 14% observed in the present study. In our study, all patients received prophylactic anticoagulation since admission.
Demographics, clinical data, and cardiovascular assessments, including ECG and the measurement of relevant biomarkers such as troponin, were included in our study. This study proposes an innovative approach that estimates the risk of ICU admission and death in the short term and provides explanatory explanations to translate key factors such as age, troponin positivity, and ECG changes into understandable and actionable clinical language. This will lead to improved clinical decision-making, optimization of ICU bed allocation in resource-constrained settings, and increased acceptance and trust of the model results by the healthcare team.
5.1. Conclusions
This comprehensive study of 216 COVID-19 patients highlights key cardiovascular complications linked to adverse outcomes, such as ICU admission and mortality. It confirms that advanced age and positive troponin levels are strong predictors of severity and death, aligning with existing research. The study also identified prevalent electrocardiographic abnormalities like sinus tachycardia and rhythm disturbances, along with significant reductions in ventricular function, especially in severe cases. Fluid buildup, such as pericardial and bilateral pleural effusions, was associated with a worse prognosis, while vascular issues like PTE, particularly sub-segmental types, significantly increased mortality risks. Overall, these findings emphasize the importance of personalized cardiovascular monitoring in COVID-19 management to improve risk assessment and clinical outcomes.
5.2. Limitations
This study provides valuable insights into COVID-19's cardiovascular impacts but has several limitations. Its retrospective, single-center design restricts causal inferences and may involve incomplete data, especially for subclinical conditions. The findings may not be generalizable due to selection bias, as only hospitalized patients were included, excluding milder cases, and recent data sharing restrictions limit transparency. Unmeasured confounders, like socioeconomic factors and treatment variations, could influence results. Diagnostic tools used may have varying accuracy, and the study's design prevents establishing a direct temporal link between COVID-19 and cardiovascular outcomes. Additionally, evolving clinical practices mean the findings may not reflect current care standards, highlighting the need for prospective, multicenter studies with broader data collection to validate and expand these observations.