1. Background
The emergence of the novel coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has created unprecedented challenges for global health systems (1). Since its identification in late 2019, the virus has resulted in significant morbidity and mortality worldwide. As of mid-2023, over 600 million confirmed cases and more than 6 million deaths have been reported, highlighting the urgent need for effective preventive measures (1, 2).
Vaccination has emerged as a critical strategy for mitigating the impact of COVID-19. The rapid development and deployment of vaccines have been instrumental in reducing the incidence of severe disease, hospitalizations, and deaths associated with SARS-CoV-2 infection (3). Various vaccine platforms, including inactivated virus vaccines, viral vector vaccines, and protein subunit vaccines, have received emergency use authorization and have been administered globally (4).
In Iran, the national immunization program has incorporated multiple vaccine types to address the pandemic effectively. This includes internationally developed vaccines such as Sinopharm (BBIBP-CorV), Sputnik V, and AstraZeneca (ChAdOx1-S), alongside domestically produced vaccines like COVIran Barekat and Pasteurcovac (5-7). The diversity of vaccines utilized reflects efforts to ensure broad immunization coverage amid global supply constraints (4).
Assessing the effectiveness of these vaccines in real-world settings, particularly concerning hospitalization and mortality outcomes, is crucial. Studies have shown that immunization significantly reduces the risk of severe COVID-19 outcomes. For example, a nationwide cross-sectional study in Iran indicated that post-immunization, COVID-19-related mortality decreased by approximately 50% compared to the pre-immunization period, despite a rise in hospital admissions during the Delta variant surge (5, 8).
The COVID-19 immunization markedly lowers the risk of hospitalization and death, especially among vulnerable populations. This protective effect arises from the stimulation of neutralizing antibodies and T-cell responses. Research has demonstrated high effectiveness, with the BNT162b2 vaccine reducing severe illness by 93%. Even a single dose of ChAdOx1-S (AstraZeneca) provides strong protection, particularly for older adults. However, vaccine effectiveness may diminish over time, and the emergence of new variants like Delta and Omicron underscores the importance of booster shots. Comorbidities continue to pose risks, emphasizing the need for targeted protection strategies. Understanding immunization status is essential for predicting severe outcomes (5-9).
Nevertheless, vaccine effectiveness can vary based on several factors, including the type of vaccine, the number of doses administered, the intervals between doses, the emergence of new variants, and individual patient characteristics such as age and comorbidities. Thus, evaluating vaccine performance in specific populations and settings is vital for informing public health strategies (10, 11).
2. Objectives
This study aims to investigate the association between COVID-19 immunization status, including the number of doses received, the type of vaccine administered, and hospitalization outcomes, among patients with confirmed SARS-CoV-2. This research seeks to elucidate the protective effects of immunization against severe disease and death in a real-world context.
3. Methods
This cross-sectional study involved 5,600 patients with confirmed COVID-19 (positive RT-PCR test) in Dezful, southwest Iran, from June to September 2021. The study population consisted of all confirmed cases during this period, using a census sampling method that included all eligible patients without random selection. Patients were grouped into three clinical outcomes: (1) Outpatients who did not require hospitalization, (2) hospitalized patients (including those in general wards and ICUs), and (3) deceased patients.
Data were collected from the Health Deputy of Dezful University of Medical Sciences and the hospital information system (HIS) of Ganjavian Hospital, the primary COVID-19 referral center in the city. Inclusion criteria were: (1) Confirmed SARS-CoV-2 infection via positive PCR, and (2) availability of immunization and hospitalization status. Patients with incomplete records for these critical variables were excluded from the analysis.
Vaccination data included the number of doses received (0 to 3) and the type of vaccine administered, which included Sinopharm, Sputnik V, AstraZeneca, COVIran Barekat, Bharat Biotech, and Pasteurcovac (PastoCoVac). Comorbidities were also documented, including cardiovascular disease, diabetes, hypertension, chronic respiratory disease, and kidney disorders.
Clinical data encompassed demographic variables (age, sex, education level), presence of symptoms (e.g., cough, dyspnea, headache, ageusia, myalgia), need for mechanical ventilation, ICU admission, and outcome (recovery or death). Disease severity was classified according to national COVID-19 treatment guidelines based on respiratory distress, oxygen saturation levels, and imaging findings.
Statistical analyses were conducted using Stata SE version 13. Descriptive statistics (means, standard deviations, frequencies, and percentages) summarized the data. Before and after comparisons were made using the paired t-test. For categorical variables with small subgroup sizes, Fisher’s exact test was considered where appropriate. For the continuous variable (age), normality assumptions were verified before applying t-tests. Alternative approaches were used if assumptions were violated. To evaluate vaccine effectiveness while adjusting for confounders, multivariable logistic regression analyses were performed, and adjusted odds ratios (OR) with standard errors (SE) and 95% confidence intervals (CI) were reported.
This study received approval from the Ethics Committee of Dezful University of Medical Sciences, approval ID: IR.DUMS.REC.1401.013. All data were anonymized before analysis, and patient confidentiality was strictly upheld throughout the study.
4. Results
The study involved 5,600 COVID-19 patients, with a nearly equal gender distribution: 2,786 males (49.75%) and 2,814 females (50.25%). Patient ages ranged from 2 to 105 years, with a mean age of 50.9 ± 18.53 years. Most patients (4,906, 87.61%) were managed as outpatients, while 694 (12.39%) required hospitalization. Clinical outcomes indicated that 4,864 patients (86.89%) were discharged, 655 (11.70%) died, and 79 (1.41%) remained hospitalized. Vaccination data showed that 4,532 patients (80.93%) were unvaccinated, while 454 (8.11%), 500 (8.93%), and 114 (2.04%) had received one, two, or three doses, respectively. The mean age of hospitalized patients was 52.59 ± 18.29 years, significantly older than that of the outpatients, whose mean age was 39.01 ± 15.53 years.
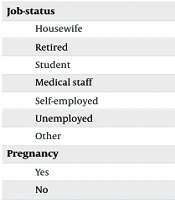
According to Table 1, hospitalization rates varied by occupation, with medical staff showing the highest admission rate (63.97%), while homemakers had the lowest (6.11%). Comorbidities revealed unexpected patterns — patients with cardiovascular disease (3.52%) or diabetes (3.77%) had lower hospitalization rates compared to those without (13.80% and 13.88%, respectively). Symptom analysis showed that patients presenting with headache (19.85%) or myalgia (14.15%) were more likely to be hospitalized than those with respiratory symptoms like cough (11.62%) or shortness of breath (5.42%).
| Variables | Values | Inpatient | Outpatient |
|---|---|---|---|
| Sex | |||
| Male | 2,786 (49.75) | 349 (12.53) | 2,437 (87.47) |
| Female | 2,814 (50.25) | 345 (12.26) | 2,469 (87.74) |
| Job-status | |||
| Housewife | 2,046 (36.54) | 125 (6.11) | 1,921 (93.89) |
| Retired | 989 (17.66) | 48 (4.85) | 941 (95.15) |
| Student | 92 (1.64) | 32 (34.78) | 60 (65.22) |
| Medical staff | 358 (6.39) | 229 (63.97) | 129 (36.03) |
| Self-employed | 950 (16.96) | 93 (9.79) | 857 (90.21) |
| Unemployed | 142 (2.54) | 11 (7.75) | 131 (92.25) |
| Other | 1,023 (18.27%) | 156 (15.25) | 867 (84.75) |
| Pregnancy | |||
| Yes | 95 (1.70) | 13 (13.68) | 82 (86.32) |
| No | 5,505 (98.30) | 681 (12.37) | 4,824 (87.63) |
| Cardiovascular disease | |||
| Yes | 768 (13.71) | 27 (3.52) | 741 (96.48) |
| No | 4,832 (86.29) | 667 (13.80) | 4,165 (86.20) |
| Pulmonary disease | |||
| Yes | 63 (1.13) | 3 (4.76) | 60 (95.24) |
| No | 5,537 (98.87) | 691 (12.48) | 4,846 (87.52) |
| Diabetes | |||
| Yes | 823 (14.70) | 31 (3.77) | 792 (96.23) |
| No | 4,777 (85.30) | 663 (13.88) | 4,114 (86.12) |
| Kidney disease | |||
| Yes | 144 (2.57) | 9 (6.25) | 135 (93.75) |
| No | 5,456 (97.43) | 685 (12.55) | 4,771 (87.45) |
| Chills | |||
| Yes | 1,086 (19.39) | 141 (12.98) | 945 (87.02) |
| No | 4,514 (80.61) | 553 (12.25) | 3,961 (87.75) |
| Myalgia | |||
| Yes | 1,816 (32.43) | 257 (14.15) | 1,559 (85.85) |
| No | 3,784 (67.57) | 437 (11.55) | 3,347 (88.45) |
| Cough | |||
| Yes | 3,271 (58.41) | 380 (11.62) | 2,891 (88.38) |
| No | 2,329 (41.59) | 314 (13.48) | 2,015 (86.52) |
| Shortness of breath | |||
| Yes | 2,988 (53.36) | 162 (5.42) | 2,826 (94.58) |
| No | 2,612 (46.64) | 532 (20.37) | 2,080 (79.63) |
| Headache | |||
| Yes | 962 (17.18) | 191 (19.85) | 771 (80.15) |
| No | 4,638 (82.82) | 503 (10.85) | 4,135 (89.15) |
| Ventilator use | |||
| Yes | 86 (1.54) | 5 (5.81%) | 81 (94.19) |
| No | 4,814 (98.46) | 595 (12.36) | 4,219 (87.64) |
a Values are expressed as No. (%).
Table 2 shows a significant inverse relationship between the number of vaccine doses received and the likelihood of hospitalization (P < 0.001). Unvaccinated patients had the highest hospitalization rate at 89.81%, while those who received three doses had the lowest rate at 61.40%. Furthermore, patients who received any vaccine dose — first, second, or third — were significantly less likely to be hospitalized compared to unvaccinated individuals. The type of vaccine also influenced hospitalization rates, with vaccines like Sputnik V and Bharat Biotech generally leading to lower rates than others, such as Sinopharm.
| Variables | Values | Inpatient | Outpatient | P-Value |
|---|---|---|---|---|
| Number of vaccine doses | 0.001 | |||
| 0 dose | 4532 (80.93) | 4070 (89.81) | 462 (10.19) | |
| 1 dose | 454 (8.11) | 404 (88.99) | 50 (11.01) | |
| 2 doses | 500 (8.93) | 362 (72.40) | 138 (27.60) | |
| 3 doses | 114 (2.04) | 70 (61.40) | 44 (38.60) | |
| Vaccination state | ||||
| First dose | 0.001 | |||
| Received | 1129 (20.18) | 883 (78.21) | 246 (21.79) | |
| Not received | 4471 (79.82) | 4023 (89.98) | 448 (10.02) | |
| Second dose | 0.001 | |||
| Received | 645 (11.52) | 455 (70.54) | 190 (29.46) | |
| Not received | 4955 (88.48) | 4451 (89.83) | 504 (10.17) | |
| Third dose | < 0.001 | |||
| Received | 114 (2.04) | 70 (61.40) | 44 (38.60) | |
| Not received | 5486 (97.96) | 4836 (88.15) | 650 (11.85) | |
| Vaccine type | ||||
| First dose | 0.001 | |||
| Sinopharm | 762 (67.49) | 668 (87.66) | 94 (12.34) | |
| Sputnik V | 152 (13.46) | 60 (39.47) | 92 (60.53) | |
| AstraZeneca | 107 (9.48) | 73 (68.22) | 34 (31.78) | |
| COVIran Barekat | 88 (7.79) | 73 (82.95) | 15 (17.05) | |
| Bharat Biotech | 20 (1.77) | 9 (45.00) | 11 (55.00) | |
| Second dose | 0.001 | |||
| Sinopharm | 398 (61.71) | 333 (83.67) | 65 (16.33) | |
| Sputnik V | 142 (22.02) | 58 (40.85) | 84 (59.15) | |
| AstraZeneca | 53 (8.22) | 31 (58.49) | 22 (41.51) | |
| COVIran Barekat | 34 (5.27) | 24 (70.59) | 10 (29.41) | |
| Bharat Biotech | 18 (2.79) | 9 (50.00) | 9 (50.00) | |
| Third dose | 0.002 | |||
| Sinopharm | 52 (45.61) | 41 (78.85) | 11 (21.15) | |
| Sputnik V | 8 (7.02) | 6 (75.00) | 2 (25.00) | |
| AstraZeneca | 33 (28.95) | 15 (45.45) | 18 (54.55) | |
| COVIran Barekat | 3 (2.63) | 2 (66.67) | 1 (33.33) | |
| PastoCovac | 18 (15.79) | 6 (33.33) | 12 (66.67) |
aValues are expressed as No. (%).
Among the 5,600 COVID-19 patients, a total of 655 individuals (11.70%) died. The mean age at the time of death was 66.09 ± 16.02 years. Additionally, 55.27% (n = 362) of the deceased were male.
Table 3 shows that a significant majority (81.53%) of individuals had not received any vaccine doses, while only 0.76% had received three doses. Among those with known vaccine types, Sinopharm was the most commonly administered as the first dose (79.39%), followed by AstraZeneca (12.21%). Similar patterns were observed for the second and third doses. Notably, only 20% of deceased patients had received at least one dose, and less than 1% had completed the three-dose immunization series (Table 4).
| Variables | Values | P-Value |
|---|---|---|
| Number of vaccine doses | 0.0001 | |
| 0 dose | 534 (81.53) | |
| 1 dose | 56 (8.54) | |
| 2 doses | 60 (9.16) | |
| 3 doses | 5 (0.76) | |
| Vaccine type | ||
| First dose | 0.009 | |
| Sinopharm | 104 (79.39) | |
| Sputnik V | 1 (0.76) | |
| AstraZeneca | 16 (12.21) | |
| COVIran Barekat | 10 (7.63) | |
| Second dose | 0.026 | |
| Sinopharm | 58 (81.69) | |
| AstraZeneca | 10 (14.08) | |
| COVIran Barekat | 3 (4.23) | |
| Third dose | 0.032 | |
| Sinopharm | 3 (60.0) | |
| AstraZeneca | 1 (20.0) | |
| COVIran Barekat | 1 (20.0) | |
| Vaccination status | ||
| Stage 1 | 0.0003 | |
| Yes | 131 (20.0) | |
| No | 524 (80.0) | |
| Stage 2 | 0.019 | |
| Yes | 71 (10.84) | |
| No | 584 (89.16) | |
| Stage 3 | 0.001 | |
| Yes | 5 (0.76) | |
| No | 650 (99.24) |
aValues are expressed as No. (%).
| Variables | Adjusted OR | SE | 95% CI | P-Value |
|---|---|---|---|---|
| Age (per year increase) | 1.04 | 0.01 | 1.03 - 1.06 | < 0.001 |
| Male sex | 1.32 | 0.12 | 1.10 - 1.59 | 0.002 |
| ≥ 1 Comorbidity | 1.89 | 0.20 | 1.54 - 2.32 | 0.001 |
| Vaccination | ||||
| 1 dose | 0.72 | 0.07 | 0.60 - 0.86 | 0.001 |
| 2 doses | 0.55 | 0.06 | 0.45 - 0.68 | 0.001 |
| 3 doses | 0.38 | 0.11 | 0.22 - 0.65 | 0.001 |
| Sinopharm vaccine | 0.81 | 0.11 | 0.65 - 1.01 | 0.064 |
| AstraZeneca vaccine | 0.63 | 0.13 | 0.45 - 0.88 | 0.007 |
| Barekat vaccine | 0.70 | 0.17 | 0.41 - 1.18 | 0.182 |
Abbreviations: OR, odds ratios; SE, standard errors; CI, confidence intervals.
a Adjusted OR with 95% CI and P-values based on multivariable logistic regression analysis.
5. Discussion
The results of this study provide strong evidence of the link between COVID-19 immunization and clinical outcomes, particularly hospitalization and mortality, in a real-world setting in southwest Iran. Our analysis revealed a significant inverse relationship between the number of vaccine doses received and the likelihood of hospitalization and death. Unvaccinated individuals were disproportionately represented among hospitalized and deceased patients, while fully vaccinated individuals, especially those who received three doses, experienced considerably better clinical outcomes. Globally, immunization has been shown to stimulate both humoral and cellular immunity, reducing infection rates and the severity of disease in breakthrough cases (12, 13). In this study, 89.81% of hospitalized patients and 81.53% of those who died were unvaccinated, underscoring the critical protective role of immunization. This aligns with findings from large-scale cohort studies in the United States and Europe, where unvaccinated individuals had hospitalization and death rates 5 to 10 times higher than their vaccinated counterparts (14, 15).
Our data specifically highlight a dose-response effect: Individuals who received two or more vaccine doses had significantly lower hospitalization risks. This finding supports previous research indicating that two-dose regimens provide strong protection, while booster doses are vital for maintaining immunity, particularly against variants like Delta and Omicron that partially evade immune responses (16, 17). For instance, a nationwide cohort study from Sweden reported that a third (booster) dose of an mRNA COVID-19 vaccine, including BNT162b2, was highly effective in preventing severe COVID-19 outcomes, such as hospitalization and death, particularly among older adults (18).
In terms of vaccine types, while Sinopharm was the most commonly administered vaccine among deceased patients, this likely reflects its widespread use in the Iranian immunization program rather than an inherent lack of efficacy. Nonetheless, studies suggest that inactivated vaccines may produce a weaker neutralizing antibody response compared to mRNA vaccines, especially in elderly individuals or those with comorbidities (5, 19). Our findings indicate that both the type of vaccine and the number of doses received are crucial for assessing vaccine effectiveness in real-world populations.
Interestingly, our analysis found that specific symptoms (e.g., myalgia and headache) were more predictive of hospitalization than traditional respiratory symptoms, warranting further investigation. Additionally, the unexpectedly lower hospitalization rates among individuals with comorbidities like cardiovascular disease and diabetes contradict global data, potentially due to underreporting or differences in healthcare access and behavior in this population (9, 20). These observations underscore the need for adjusted multivariate analyses to account for confounding variables.
Our findings are consistent with evidence from Iran and other middle-income countries, demonstrating that COVID-19 vaccines significantly reduce illness severity and the burden on hospital systems (5, 7). Given the limited ICU capacity in many areas, the decrease in hospitalizations due to immunization has important public health implications beyond individual protection. By reducing the number of severe cases, immunization helps preserve healthcare resources and mitigates the strain on overwhelmed systems.
From a policy perspective, the relatively low percentage of fully vaccinated or boosted individuals among the deceased highlights the urgent need to improve vaccine coverage and booster uptake (8). Public health campaigns should particularly focus on high-risk groups, such as older adults and those with chronic diseases, while addressing vaccine hesitancy through education and trust-building initiatives (21).
This study possesses several strengths, including a population-based design, comprehensive clinical data, and the inclusion of multiple vaccine types. Nonetheless, certain limitations must be acknowledged. The retrospective nature of the study, the lack of genomic sequencing for SARS-CoV-2 variants, and the absence of long-term follow-up restrict causal inference. Future research employing longitudinal designs and multivariate models will be instrumental in elucidating the complex relationships among immunization status, variant type, comorbidities, and disease progression.
Our findings underscore the critical role of COVID-19 immunization in preventing hospitalization. The observed dose-dependent protection highlights the importance of completing primary immunization schedules and administering booster doses. Public health initiatives should prioritize expanding access, enhancing vaccine uptake, and educating communities about the life-saving benefits of immunization, particularly in resource-constrained settings.
5.1. Conclusions
This study provides compelling evidence that COVID-19 immunization significantly decreases the risk of hospitalization and death in real-world situations. A strong inverse relationship was found between the number of vaccine doses received and the severity of clinical outcomes, highlighting the importance of completing both primary and booster immunization schedules. Most patients who died were unvaccinated, while those who received two or more doses had considerably better outcomes. These results underscore the vital role of national immunization programs in reducing the impact of COVID-19 and stress the need for ongoing public health initiatives to enhance vaccine coverage, particularly among high-risk populations. Future strategies should focus on administering booster doses, monitoring vaccine effectiveness against various variants, and implementing targeted interventions for vulnerable groups.