1. Background
The World Health Organization (WHO) defines diabetes as a condition in which the pancreas fails to produce sufficient insulin, or the body is unable to effectively utilize the insulin produced, resulting in impaired blood glucose regulation (1). The global burden of diabetes has risen dramatically over the past three decades. In 2000, an estimated 171 million people were living with diabetes; by 2021, this number had increased to 529 million, and projections suggest it will reach 1.31 billion by 2050 (2). Neuropathic pain, which affects approximately 7 - 10% of the global population, poses a substantial challenge to quality of life, healthcare systems, and socioeconomic productivity (3, 4). Major causes of neuropathic pain include diabetes mellitus, chemotherapy-induced neuropathy, and post-herpetic neuralgia, with diabetic peripheral neuropathy (DPN) affecting 30 - 50% of individuals with diabetes (5). Reports indicate that up to 80% of diabetes-related healthcare costs are attributable to complications. Among these, diabetic neuropathy contributes significantly through increased hospitalization, amputations, and reduced economic productivity (6).
A 2018 study in Iran estimated that approximately $4 billion in direct and indirect costs are allocated annually to diabetes care, with a considerable proportion related to complications such as neuropathy (7). Type 2 diabetes mellitus (T2DM) is a complex metabolic disorder characterized not only by insulin resistance and hyperglycemia but also by chronic low-grade inflammation that profoundly affects the immune system. Dysregulated immune responses, including elevated pro-inflammatory cytokines such as TNF-α and IL-6, contribute to endothelial dysfunction and peripheral nerve injury, ultimately leading to diabetic neuropathy (8). Despite advances in treatment, no definitive cure for neuropathic pain has been identified. Strict blood glucose control remains the most fundamental and effective strategy for managing diabetic neuropathy. However, given the multifactorial nature of the disease, treatment typically requires a combination of approaches aimed at alleviating symptoms, slowing disease progression, and preventing complications (9). This suggests that factors beyond hyperglycemia contribute to the development of diabetic neuropathy (10).
Vitamin D, a fat-soluble secosteroid hormone best known for its essential role in calcium homeostasis and bone metabolism, has attracted growing interest for its involvement in a wide range of physiological processes beyond skeletal health (11). Recent studies have highlighted the role of vitamin D as a key immunomodulatory factor that influences both innate and adaptive immunity. Vitamin D enhances antimicrobial peptide production, promotes regulatory T-cell (Treg) activity, and suppresses pro-inflammatory pathways, thereby restoring immune balance (12-14). Moreover, clinical evidence suggests that vitamin D deficiency is strongly associated with increased risk and severity of DPN, while supplementation may alleviate neuropathic pain and improve nerve conduction (15). These findings underscore the importance of investigating vitamin D as a potential adjunctive therapy in the management of T2DM and its complications. In addition, vitamin D may enhance the production of endogenous opioid peptides and regulate descending pain inhibitory pathways, thereby reducing pain perception (16, 17).
Given the high global prevalence of vitamin D deficiency, particularly among populations at increased risk of neuropathic pain, such as individuals with diabetes and older adults — the potential therapeutic role of vitamin D in pain management is of considerable importance (18). Observational studies have reported associations between low serum vitamin D levels and an increased risk of chronic pain conditions, including neuropathic pain (19, 20). Furthermore, some clinical trials suggest that vitamin D supplementation may reduce pain intensity and improve functional outcomes in patients with neuropathic pain (21, 22). However, findings remain inconsistent, as other studies have failed to demonstrate significant benefits, possibly due to differences in study design, patient populations, vitamin D dosage, and treatment duration (23).
2. Objectives
A comprehensive and critical evaluation of existing evidence is necessary to clarify the role of vitamin D in managing neuropathic pain. In light of this, the present study was conducted to investigate the effect of vitamin D administration on the severity of neuropathic pain in diabetic patients in Dezful city in 2025.
3. Methods
3.1. Study Design
This study was designed as a quasi-experimental (before-and-after) trial. A total of 19 patients with type 2 diabetes and painful diabetic neuropathy were recruited from the Dezful Diabetes Research Center between November 14, 2024, and May 22, 2025. To minimize bias and enhance the validity of findings, this quasi-experimental study utilized a single-blind design in which both participants and outcome assessors remained unaware of the study’s specific objectives and the timing of the intervention.
3.2. Inclusion Criteria
Participants were eligible if they met the following criteria: Diagnosis of type 2 diabetes; possession of a medical record at the Dezful Diabetes Research Center; willingness to participate in the study; presence of diabetic neuropathy.
3.3. Diagnostic Tools
Diabetic neuropathy was assessed using the Michigan Neuropathy Screening Instrument (MNSI) and electromyography–nerve conduction velocity (EMG-NCV).
The MNSI consists of two components: A 15-item questionnaire and a physical examination. It is a validated tool for screening peripheral neuropathy in diabetic patients.
Michigan Neuropathy Screening Instrument Questionnaire: The questionnaire included 15 yes/no questions. Thirteen items assessed symptoms of DPN, one item assessed peripheral vascular disease, and one item assessed general weakness. The questions focused on sensations in the feet and lower legs, including: Numbness in the legs and/or feet; burning sensations in the legs and/or feet; hypersensitivity of the feet to touch; muscle cramps in the legs and/or feet; prickling sensations in the legs or feet; pain when bed covers touch the skin; ability to distinguish hot from cold water in the shower or bath; history of open sores on the foot; previous diagnosis of diabetic neuropathy by a physician; generalized weakness most of the time; worsening of symptoms at night; leg pain during walking; ability to sense the feet while walking; presence of dry, cracked skin on the feet; history of amputation.
Michigan Neuropathy Screening Instrument Physical Examination: After completing the questionnaire, patients underwent a structured clinical examination (Table 1).
| Parameter | Normal (0) | Mild/Reduced (0.5) | Abnormal (1) |
|---|---|---|---|
| Foot appearance | 0 = Normal | - | 1 = Deformity, dry skin, calluses, infection, or cracks |
| Wounding | 0 = Present | - | 1 = Absent |
| Monofilament test | 0 = Normal (≥ 8 sites perceived) | 0.5 = Partial (1 - 7 sites perceived) | 1 = No response |
| Vibration perception | 0 = Normal | 0.5 = Reduced | 1 = Absent |
Foot inspection: Feet were examined for deformities, dry skin, calluses, infections, and ulcers. One point was assigned for any abnormality, with an additional point for the presence of an ulcer.
Vibration sensation: Tested using a 128 Hz tuning fork. If the patient could not perceive vibration, one point was assigned. A score of 0 indicated normal sensation, while 0.5 indicated mild to moderate impairment.
Monofilament test: If ≥ 8 designated sites were perceived, a score of 0 was assigned. If 1 - 7 sites were perceived, a score of 0.5 was assigned. No response resulted in a score of 1.
All positive responses and abnormal findings from the physical examination were recorded on the questionnaire form.
In both the MNSI questionnaire and physical examination, higher scores indicate a greater risk of neuropathy. Diabetic peripheral neuropathy was diagnosed when the physical examination score exceeded 2 and the questionnaire score exceeded 4.
Following diagnosis with the MNSI, patients with serum vitamin D levels below 30 ng/mL were referred for nerve conduction studies (NCS). Nerve conduction studies is a key diagnostic tool for evaluating the severity of diabetic neuropathy, as it measures peripheral nerve and muscle function. Parameters assessed included motor nerve amplitude, sensory nerve amplitude, motor nerve conduction velocity, and F-wave latency (FML). All tests were performed by a neurologist, and diabetic neuropathy was confirmed in selected patients.
3.4. Exclusion Criteria
Patients were excluded if they: Lived outside Dezful and were unlikely to attend follow-up visits; had lower limb amputation; were pregnant or lactating; experienced acute, severe lower limb pain requiring analgesic injections; had other causes of leg pain (e.g., peripheral arterial disease or infections); had used oral or injectable vitamin D, calcium, or multivitamins within the past 6 months; had comorbidities such as renal failure, hepatic failure, heart failure, inflammatory arthritis, hyperparathyroidism, alcohol addiction, or malnutrition
3.5. Study Population
A total of 95 patients were initially evaluated. Of these, 54 did not meet the inclusion criteria, 5 were excluded during NCS testing, and 17 declined participation. Ultimately, 19 patients were enrolled in the trial.
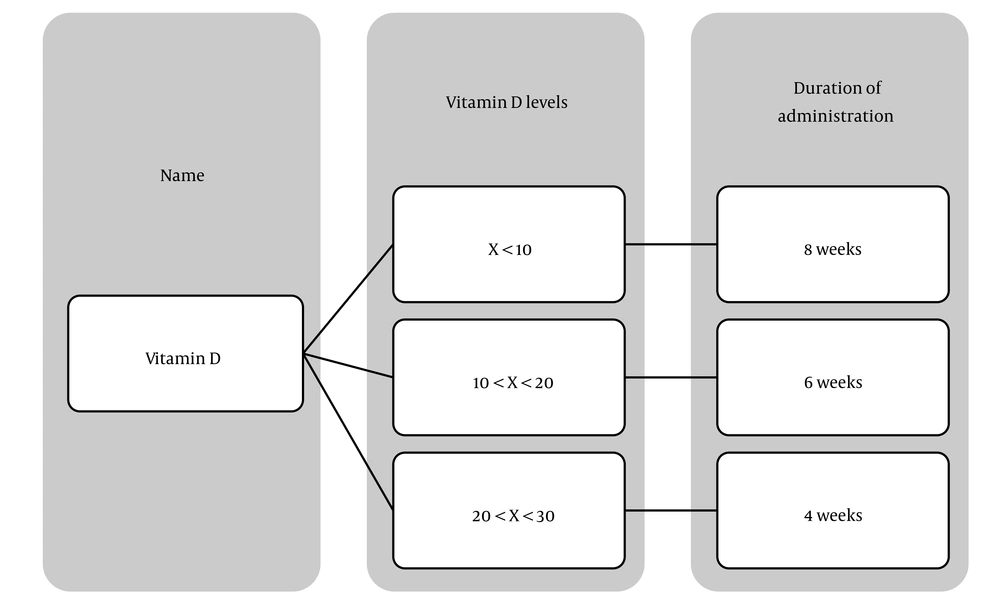
All participants were regularly followed up at the Dezful Diabetes Research Center. A detailed medical history was recorded, and a physical examination was performed for each patient. Based on baseline vitamin D levels, patients received weekly oral supplementation of 50,000 IU vitamin D3 for a minimum of 4 weeks and a maximum of 8 weeks (Figure 1).
3.6. Intervention and Data Collection
Patients received 50,000 international units (IU) of vitamin D3 weekly in the form of oral tablets. Neuropathy assessments were conducted both before and after the intervention using the MNSI, which included the 15-item questionnaire and the clinical examination.
In addition, blood samples were collected after 8 hours of fasting to measure fasting plasma glucose (FPG), HbA1c, and 25(OH) vitamin D levels at baseline and following the intervention.
3.7. Statistical Analysis
Data analysis was performed using SPSS software version 26. Quantitative variables were expressed as mean ± standard deviation (SD), while qualitative variables were reported as frequency (percentage).
The Kolmogorov-Smirnov test was used to assess the normality of data distribution.
For normally distributed data, paired t-tests were applied to compare pre- and post-intervention values.
For non-normally distributed data, the Wilcoxon signed-rank test was used.
A P-value < 0.05 was considered statistically significant.
Based on the data reported by Ghadiri-Anari et al., the effect size for the MNSI score was first calculated by comparing pre- and post-intervention values. Using this effect size, the required sample size was estimated with the pwr package in R (version 4.2.2), assuming a type I error rate of 0.05 and a statistical power of 90%. This calculation yielded a minimum of 19 participants per group, encompassing both pre- and post-intervention groups (24).
3.8. Ethical Considerations
The study was approved by the Research Ethics Committee of Dezful University of Medical Sciences (ethics code: IR.DUMS.REC.1403.057). Written informed consent was obtained from all participants prior to enrollment.
4. Results
In this study, 19 patients with a mean age of 60.38 ± 10.38 years were examined, including 8 women (42.1%) and 11 men (57.9%) (Table 2). The mean Body Mass Index (BMI) of participants was 26.71 ± 4.87. Most individuals were either self-employed (42.1%) or homemakers (36.8%). The majority (84.2%) were non-smokers. Regarding medical history, 63.2% of patients had diabetes only, 26.3% had diabetes with hypertension, and 10.5% had diabetes with heart disease. The mean duration of diabetes was 13.05 ± 5.92 years, while the mean duration of neuropathy was 3.21 ± 3.19 years.
| Characteristic | Value |
|---|---|
| Age (y) | 60.00 ± 10.38 |
| Gender | |
| Male | 8 (42.1) |
| Female | 11 (57.9) |
| BMI (kg/m²) | 26.71 ± 4.87 |
| Occupation | |
| Employed | 8 (42.1) |
| Retired | 3 (15.8) |
| Housewife | 7 (36.8) |
| Laborer | 1 (5.2) |
| Smoking status | |
| Yes | 3 (15.8) |
| No | 16 (84.2) |
| Medical history | |
| Diabetes and Hypertension | 12 (63.2) |
| Other | 5 (26.2) |
| None | 2 (10.8) |
| Duration of diabetes (y) | 13.05 ± 5.92 |
| Duration of neuropathy (y) | 3.21 ± 3.19 |
a Values are expressed as No (%) or mean ± SD.
As shown in Table 3, baseline biochemical parameters were as follows: Mean vitamin D level 18.58 ± 5.97 ng/mL, HbA1c 8.63 ± 1.13%, creatinine 1.04 ± 0.243 mg/dL, fasting blood sugar 198.74 ± 65.22 mg/dL, motor nerve amplitude 2.04 ± 2.40, sensory nerve amplitude 7.24 ± 24.36, motor nerve conduction velocity 46.52 ± 22.38, and FML 24.88 ± 25.07. These findings provide preliminary insights into the potential effect of vitamin D supplementation on neuropathic pain severity in diabetic patients.
| Parameter | Value |
|---|---|
| Vitamin D (ng/mL) | 18.58 ± 5.97 |
| HbA1c (%) | 8.63 ± 1.13 |
| Creatinine (mg/dL) | 1.04 ± 0.24 |
| Fasting Blood Sugar (mg/dL) | 198.74 ± 65.22 |
| Motor Nerve Latency (ms) | 2.04 ± 2.40 |
| Sensory Nerve Amplitude (µV) | 7.24 ± 24.36 |
| Motor Nerve Conduction Velocity (m/s) | 46.52 ± 22.38 |
| FML | 24.88 ± 25.07 |
a Values are expressed as mean ± SD.
The results of the Michigan questionnaire demonstrated significant changes in the reporting of peripheral neuropathy symptoms following vitamin D supplementation (Table 4). Overall, most patients experienced a reduction in symptoms after the intervention. Sensory symptoms, including numbness (decreasing from 89.5% to 63.2%), tingling (from 94.7% to 63.2%), and hypersensitivity to touch (from 30.5% to 10.2%), showed marked improvement. Pain-related symptoms also declined, with burning pain decreasing from 68.4% to 30.3% and pain during walking from 30.3% to 10.4%. Furthermore, functional impairments such as difficulty detecting water temperature and reduced foot sensation while walking improved, with prevalence decreasing from 89.5% to 70.9%.
| Row | Question | Before taking Vitamin D | After taking Vitamin D | ||
|---|---|---|---|---|---|
| Yes | No | Yes | No | ||
| 1 | Do you experience numbness in one or both legs? | 17 (89.47) | 2 (10.53) | 12 (63.16) | 7 (36.84) |
| 2 | Have you ever had burning pain in your leg or legs? | 13 (68.42) | 6 (31.58) | 5 (26.32) | 14 (73.68) |
| 3 | Have you experienced muscle cramps in your leg or legs? | 11 (57.89) | 8 (42.11) | 6 (31.58) | 13 (68.42) |
| 4 | Are your feet very sensitive to touch? | 4 (21.05) | 15 (78.95) | 1 (5.26) | 18 (94.74) |
| 5 | Does it hurt when the bedsheet touches your foot? | 1 (5.26) | 18 (94.74) | 0 (0.00) | 19 (100.00) |
| 6 | Have you ever had open sores on your feet? | 8 (42.11) | 11 (57.89) | 8 (42.11) | 11 (57.89) |
| 7 | Has anyone ever told you that you have neuropathy? | 0 (0.00) | 19 (100.00) | 0 (0.00) | 19 (100.00) |
| 8 | Is the skin on your feet so dry that it cracks? | 14 (73.68) | 5 (26.32) | 10 (52.63) | 9 (47.37) |
| 9 | Have you ever felt weakness for most of the day? | 11 (57.89) | 8 (42.11) | 9 (47.37) | 10 (52.63) |
| 10 | Do you feel tingling in one or both legs? | 18 (94.74) | 1 (5.26) | 12 (63.16) | 7 (36.84) |
| 11 | Do your symptoms worsen at night? | 14 (73.68) | 5 (26.32) | 11 (57.89) | 8 (42.11) |
| 12 | When you enter a bathtub or shower, can you distinguish hot water from cold? | 17 (89.47) | 2 (10.53) | 18 (94.74) | 1 (5.26) |
| 13 | Have you ever had an amputation? | 0 (0.00) | 19 (100.00) | 0 (0.00) | 19 (100.00) |
| 14 | Do you feel pain when walking? | 8 (42.11) | 11 (57.89) | 5 (26.32) | 14 (73.68) |
| 15 | Can you feel your foot when walking? | 17 (89.47) | 2 (10.53) | 18 (94.74) | 1 (5.26) |
a Values are expressed as No (%).
The results of the Michigan Clinical Examination before and after vitamin D supplementation in diabetic patients indicated that while some indicators improved, others showed minimal change (Table 5). Infections and skin fissures demonstrated a relative reduction following the intervention, decreasing from 36.8% to 31.6% in the right foot and from 26.3% to 10.2% in the left foot. Foot ulcers also improved, with prevalence declining from 26.3% to 15.8% on the right foot and from 21.1% to 10.5% in the left foot. In contrast, deformities in both feet remained unchanged at 15.8%, and the presence of dry, callused skin showed no meaningful improvement (73.7% in the right foot and 40.7% in the left foot).
| Category and Item | Before Vitamin D | After Vitamin D | ||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| Right foot appearance | ||||
| Presence of deformity | 3 (15.79) | 16 (84.21) | 3 (15.79) | 16 (84.21) |
| Dry skin callus | 14 (73.68) | 5 (26.32) | 14 (73.68) | 5 (26.32) |
| Infection or crack | 7 (36.84) | 12 (63.16) | 6 (31.58) | 13 (68.42) |
| Left foot appearance | ||||
| Presence of deformity | 3 (15.79) | 16 (84.21) | 3 (15.79) | 16 (84.21) |
| Dry skin callus | 13 (68.42) | 6 (31.58) | 13 (68.42) | 6 (31.58) |
| Infection or crack | 5 (26.32) | 14 (73.68) | 4 (21.05) | 15 (78.95) |
| Wound | ||||
| Right foot | 5 (26.32) | 14 (73.68) | 3 (15.79) | 16 (84.21) |
| Left foot | 4 (21.05) | 15 (78.95) | 2 (10.53) | 17 (89.47) |
a Values are expressed as No (%).
The sensory evaluation of the lower limbs using monofilament and vibration tests demonstrated significant improvements following vitamin D supplementation (Table 6). In the monofilament test, the proportion of patients with absent or reduced sensation decreased from 94.8% to 90.8% in the right leg and from 94.7% to 90.8% in the left leg. A more pronounced improvement was observed in the vibration test, where impairment declined from 63.2% to 10.4% in the right leg and from 57.9% to 40.5% in the left leg.
| Sensation Test and Foot | Before Receiving Vitamin D | After Receiving Vitamin D | ||||
|---|---|---|---|---|---|---|
| Has Sensation | Reduced/with Reinforcement Exists | No Sensation | Has Sensation | Reduced/with Reinforcement Exists | No Sensation | |
| Monofilament | ||||||
| Right foot | 1 (5.26) | 4 (21.05) | 14 (73.68) | 4 (21.05) | 10 (52.63) | 5 (26.32) |
| Left foot | 1 (5.26) | 5 (26.32) | 13 (68.42) | 4 (21.05) | 10 (52.63) | 5 (26.32) |
| Vibration | ||||||
| Right foot | 7 (36.84) | 4 (21.05) | 8 (42.11) | 11 (57.89) | 5 (26.32) | 3 (15.79) |
| Left foot | 8 (42.11) | 4 (21.05) | 7 (36.84) | 10 (52.63) | 5 (26.32) | 4 (21.05) |
a Values are expressed as No (%).
As presented in Table 7, vitamin D levels increased significantly following supplementation (P < 0.001). Concurrently, HbA1c levels decreased significantly (P = 0.006). Improvements were also observed in both the questionnaire (P < 0.001) and the Michigan Clinical Examination (P = 0.001). The mean questionnaire score decreased from 5.31 ± 1.05 to 3.47 ± 1.50, indicating a reduction in patient-reported symptoms. The mean clinical examination score decreased from 5.50 ± 2.87 to 4.21 ± 2.19, confirming improvement in clinical status.
| Variables | Before Receiving Vitamin D | After Receiving Vitamin D | P-Value |
|---|---|---|---|
| Vitamin D | 5.97 ± 18.58 | 6.62 ± 35.03 | < 0.001 |
| HbA1c | 1.16 ± 8.55 | 1.57 ± 7.81 | 0.006 |
| Michigan Questionnaire Score | 1.05 ± 5.31 | 1.50 ± 3.47 | < 0.001 |
| Michigan Clinical Examination Score | 2.87 ± 5.50 | 2.19 ± 4.21 | 0.001 |
a Values are expressed as mean ± SD.
The global prevalence of diabetes has risen sharply in recent decades, increasing from 108 million in 1980 to 463 million in 2019 (25), with projections suggesting that the number will exceed 700 million by 2045 (26). In Iran, the prevalence of diabetes among individuals aged 25 - 70 years was reported at 11.9% in 2011, representing a 35% increase compared to earlier years. By 2030, it is estimated that nearly 9.2 million Iranians will be affected by diabetes (27).
5. Discussion
The present study demonstrates significant therapeutic benefits of vitamin D supplementation in patients with DPN, with improvements observed in both subjective symptom reports and objective clinical measures. These findings are consistent with emerging evidence supporting the role of vitamin D in neuropathic pain management and peripheral nerve function, while also contributing to the growing body of literature on micronutrient interventions in diabetic complications. Improvements in MNSI scores, sensory function tests, and biochemical parameters provide compelling evidence for vitamin D as an adjunctive therapeutic option in diabetic neuropathy. Given the burden of diabetes and the lack of similar studies in the region, this investigation — conducted in Dezful in 2025 — offers novel insights into the role of vitamin D in neuropathic pain management.
The findings of this study are in agreement with recent systematic reviews and meta-analyses. For example, the reduction in MNSI scores from 5.31 ± 1.05 to 3.47 ± 1.50 (P < 0.001) parallels results from a systematic review by Basit et al., which included 364 patients across four studies and reported a 1.14% improvement in McGill Pain Questionnaire scores in favor of vitamin D supplementation (8).
Symptom-specific improvements in this study — such as reductions in numbness (from 89.5% to 63.2%), tingling (from 94.7% to 63.2%), and burning pain (from 68.4% to 26.3%) — are clinically meaningful. These results align with a meta-analysis by Zhang et al., which included 26 studies and 6,277 participants, and found that patients with diabetic neuropathy were 2.87 times more likely to have vitamin D deficiency compared to those without neuropathy (28). This suggests that the observed improvements may reflect correction of an underlying pathophysiological deficit rather than symptomatic relief alone.
In this study, weekly oral vitamin D3 supplementation (50,000 IU for 4 - 8 weeks) significantly increased serum 25 (OH) vitamin D concentrations and improved HbA1c levels. Improvements in neuropathy were confirmed through both the MNSI questionnaire and physical examination. Notably, lower vitamin D levels were associated with higher HbA1c, consistent with previous findings (24).
However, the effect of vitamin D supplementation on glycemic control remains controversial. The SUNNY trial, for example, reported that high intermittent doses of vitamin D (50,000 IU monthly for 6 months) did not improve glycemic control in patients with type 2 diabetes, despite achieving optimal vitamin D status (29). Conversely, other studies have reported significant reductions in HbA1c and neuropathic pain symptoms following high-dose vitamin D supplementation, consistent with the present findings (30).
Several studies have also highlighted the high prevalence of vitamin D deficiency in diabetic patients compared to healthy individuals, with deficiency correlating with the severity of sensory neuropathy (31). Additional evidence suggests that topical vitamin D application to neuropathy-affected areas may reduce symptoms (32). In a prospective, placebo-controlled trial, oral vitamin D supplementation increased serum vitamin D levels and reduced neuropathy symptoms, as measured by the Neuropathy Symptom Score (NSS), though no significant improvements were observed in the Neuropathy Disability Score (NDS) or NCS (33).
The therapeutic benefits observed in this study also invite comparison with other micronutrient interventions. A randomized controlled trial study, investigated vitamin B12 supplementation in 90 patients with diabetic neuropathy for over one year. Both vitamin D and B12 interventions improved neuropathy symptoms, but the B12 trial additionally demonstrated improvements in nerve conduction parameters, including sural nerve conduction velocity and action potential (P < 0.0001) (34). Unlike the B12 trial, the present study did not assess electrophysiological parameters, highlighting an important area for future research.
It is also noteworthy that the B12 trial reported worsening pain scores in the placebo group (P < 0.0001), whereas the current study lacked a control group, limiting the ability to distinguish between treatment effects and natural disease progression (34). Nevertheless, the magnitude of symptomatic improvement observed with vitamin D supplementation is comparable to that reported with B12, suggesting that both may provide therapeutic benefits through distinct mechanisms.
The significant increase in vitamin D levels (P < 0.001) and concurrent reduction in HbA1c (P = 0.006) observed in this study suggest a potential dual benefit of vitamin D supplementation in diabetic patients. This aligns with prior research indicating that vitamin D may influence glucose metabolism and insulin sensitivity by increasing serum calcium, reducing circulating free fatty acids, and improving glucose tolerance (28).
Objective improvements in neurological function were also observed. Monofilament test results improved from 94.8% to 78.9% in the right foot and from 94.7% to 78.9% in the left foot, while vibration perception also improved. These findings provide evidence of enhanced peripheral nerve function beyond subjective symptom relief (35).
Finally, a multicenter, randomized, double-blind trial evaluating intramuscular vitamin D2 supplementation in patients with type 2 diabetes and peripheral neuropathy reported improvements in both clinical symptoms and nerve conduction velocity after one year of follow-up (36). This further supports the potential role of vitamin D in improving both symptomatic and physiological aspects of diabetic neuropathy.
5.1. Conclusions
In conclusion, vitamin D supplementation appears to play a dual role in patients with type 2 diabetes by both enhancing immune regulation and reducing the severity of diabetic neuropathy. By modulating T and B lymphocyte activity, promoting regulatory T-cell (Treg) function, and suppressing pro-inflammatory cytokines such as TNF-α and IL-6, vitamin D contributes to restoring immune balance and protecting peripheral nerves (37). Evidence further indicates that vitamin D deficiency exacerbates immune dysregulation and neuropathic complications, while adequate supplementation may serve as an adjunct therapeutic strategy to improve immune competence and mitigate neurological damage in T2DM (38, 39). These findings highlight the importance of maintaining optimal vitamin D status as part of comprehensive diabetes management.
Vitamin D supplementation appears to be a promising adjunctive therapy for diabetic neuropathy. In this study of 19 diabetic patients, supplementation was associated with significant improvements in neuropathy symptoms, consistent with findings from previous clinical trials and systematic reviews. Vitamin D deficiency is a modifiable risk factor, and its correction may reduce pain, improve nerve function, and enhance quality of life in affected patients. Given its safety, affordability, and the high prevalence of deficiency among diabetic populations, routine screening and supplementation should be considered in clinical practice. However, uncertainties remain regarding the optimal dosage and the long-term benefits of vitamin D supplementation, warranting further large-scale, controlled studies.
5.2. Limitations
The most significant limitation is the absence of a control group, which prevents definitive attribution of observed improvements to vitamin D supplementation rather than natural disease progression or placebo effects. The small sample size of only 19 participants substantially limits statistical power and generalizability to broader diabetic populations, while the variable treatment duration (4 - 8 weeks based on baseline vitamin D levels) introduces inconsistent exposure periods that may confound outcomes. Additionally, the study lacks adequate control for potential confounding variables such as changes in glycemic medications, physical activity levels, or concurrent treatments, and the relatively short follow-up period prevents assessment of long-term benefits or sustainability of improvements.