1. Background
A sore throat is one of the most common reasons for pediatric visits worldwide. Viral pathogens cause 60 - 70% of cases, whereas bacterial infections, mainly group A beta-hemolytic Streptococcus (GAS), account for about 20 - 30% of childhood pharyngitis (1, 2). Some studies have shown that 37% of pharyngitis cases in children are caused by GAS (3). The GAS contributes to a diverse range of illnesses, from minor nasopharyngeal infections to life‑threatening purulent infections and immune‑mediated disorders (4, 5). In recent years, global epidemiological data show that GAS remains a major cause of pharyngitis and related complications in low- and middle-income countries (LMICs), accounting for millions of visits annually. The World Health Organization (WHO) reports that untreated or misdiagnosed streptococcal infections continue to contribute to rheumatic heart disease, particularly in children aged 5 - 15 years (6).
Beyond clinical symptoms, the socio-economic burden of misdiagnosed GAS pharyngitis and antibiotic misuse is considerable. Recent studies have emphasized that unnecessary antibiotic prescriptions increase drug resistance, health care costs, and allergic reactions, while inappropriate non-treatment may cause severe complications such as rheumatic fever and glomerulonephritis (7-10). The development of molecular diagnostic techniques such as polymerase chain reaction (PCR)-based rapid antigen detection tests (RADTs) and point-of-care platforms has improved diagnostic accuracy but remains underused in many developing regions due to cost and availability constraints (11, 12). Despite rapid diagnostic methods such as the rapid streptococcal antigen test, which demonstrates relatively good sensitivity and specificity, throat culture remains the gold standard in the diagnosis of streptococcal sore throat. These diagnostic methods are costly and not available everywhere, and follow-up of patients is difficult (13).
On the other hand, indiscriminate use of antibiotics, in addition to paying additional costs, can lead to side effects such as anaphylaxis and death. However, the lack of proper antibiotic treatment exposes patients to risks such as the spread of infection to nearby tissues and rheumatic fever (13). Although the main goal of appropriate antibiotic treatment of streptococcal pharyngitis is to prevent acute rheumatic fever and its purulent complications, clinical improvement of signs and symptoms, shortening the transmission period, and speeding up the resumption of normal activities are also important goals in the treatment of this infection. Elective treatment in streptococcal pharyngitis is injectable penicillin; however, other penicillin derivatives such as amoxicillin, macrolides, first-generation cephalosporins, and clindamycin can be used (14).
2. Objectives
This study aims to fill an important gap in regional data by comparing GAS prevalence between symptomatic and asymptomatic children in southeastern Iran. Unlike many previous studies that focused solely on symptomatic patients, we included healthy children as a control group to evaluate the rate of asymptomatic GAS carriage. This design allows better understanding of potential carriers and contributes to antibiotic stewardship strategies.
3. Methods
3.1. Study Design and Participants
This descriptive cross-sectional study was conducted over nine months, from summer to winter 2014, in the Pediatric Emergency Department of Afzalipour Hospital in Kerman, southeastern Iran.
1. Inclusion criteria: Children aged 2 - 15 years who presented with complaints of sore throat and were clinically diagnosed with bacterial pharyngitis based on fever (> 38°C), tonsillar exudate, and tender anterior cervical adenopathy.
2. Exclusion criteria: Children with other infections (e.g., otitis media, pneumonia), recent antibiotic use (within 10 days), immunodeficiency disorders, or any medication that could mask symptoms.
Eligible children were enrolled using simple random sampling after written parental consent and verbal child assent were obtained. Before antibiotic therapy, two throat swabs were collected from each child. The control group consisted of children of the same age range (2 - 15 years) who attended the same Emergency Department during the same period for non-infectious conditions such as mild trauma, headache, allergy, or abdominal pain. They were clinically healthy, had no sore throat or fever, and no antibiotic use within the past 10 days.
3.2. Data Collection and Laboratory Methods
A structured checklist was used to record demographic data, clinical findings, and examination results by a pediatric resident and confirmed by a pediatrician. Two pharyngeal swabs (one from the tonsillar surface and one from the posterior pharyngeal wall) were collected and placed in Cary Blair transport medium for culture. Samples were delivered to the reference microbiology laboratory within 24 hours. Cultures were performed on 5% sheep blood agar (HIMEDIA, India) and incubated at 37°C for 24 - 48 hours.
3.2.1. Quality Control
Laboratory procedures were standardized using Streptococcus pyogenes ATCC 19615 as a positive control. All media were validated prior to use. Duplicate readings were performed by two independent microbiologists to minimize false negatives. Colonies showing β-hemolysis, gram-positive cocci, catalase-negative, and bacitracin-sensitive (0.04-U disk, inhibition zone ≥ 10 mm) were identified as GAS. If necessary, the pyrrolidonyl arylamidase (PYR) test (MAST, Canada) was performed for confirmation. Patient data such as fever (> 38°C oral), sore throat, cough, runny nose, gastrointestinal symptoms, tonsillar swelling, palatal petechiae, and cervical adenopathy were all recorded in the checklist.
3.3. Data Analysis
After obtaining the culture results, descriptive statistics (frequency, percentage) and chi-square tests were applied to compare categorical variables. All analyses were performed using SPSS version 21, and a P-value < 0.05 was considered statistically significant.
4. Results
A total of 200 children were enrolled — 100 with pharyngitis (case group) and 100 healthy controls. In the control group, 63% were male, while in the case group 57% were male (P = 0.38). The most common age range in both groups was 6 - 10 years, with no significant age difference (P = 0.95, Table 1).
| Variables | Group | P-Value | |
|---|---|---|---|
| Case | Control | ||
| Sex | 0.38 | ||
| Male | 57 | 63 | |
| Female | 43 | 37 | |
| Age (y) | 0.95 | ||
| 2 - 5 | 35 (35) | 36 (36) | |
| 6 - 10 | 43 (43) | 41 (41) | |
| 11 - 15 | 22 (22) | 23 (23) | |
a Values are expressed as No. (%).
4.1. Seasonal Distribution
Among culture-positive pharyngitis patients, 5.6% were detected in summer, 50% in autumn, and 44.4% in winter. In controls, 12.5% were positive in summer, 37.5% in autumn, and 50% in winter. Although more positive cases occurred in cooler seasons, no statistically significant seasonal trend was observed (P = 0.14).
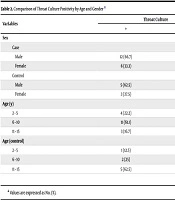
In pharyngitis patients, 18% and in healthy controls 8% had positive throat cultures for GAS (P = 0.03). The highest rate of throat culture positivity among cases was observed in the 6 - 10 years age group (61.1%), in boys (66.7%), and during autumn (50%). In controls, positivity was highest among 11 - 15 years (62.5%) and in boys (62.5%), which was significant by age (P = 0.01; Table 2). These findings suggest that school-aged children are at higher risk of both symptomatic and asymptomatic GAS carriage, particularly during cooler months.
| Variables | Throat Culture | P-Value | |
|---|---|---|---|
| + | - | ||
| Sex | |||
| Case | 0.72 | ||
| Male | 12 (66.7) | 51 (62.1) | |
| Female | 6 (33.3) | 31 (37.9) | |
| Control | 0.74 | ||
| Male | 5 (62.5) | 52 (56.5) | |
| Female | 3 (37.5) | 40 (43.5) | |
| Age (y) | 0.15 | ||
| 2 - 5 | 4 (22.2) | 32 (39) | |
| 6 - 10 | 11 (61.1) | 30 (36.5) | |
| 11 - 15 | 3 (16.7) | 20 (24.5) | |
| Age (control) | 0.01 | ||
| 2 - 5 | 1 (12.5) | 34 (37) | |
| 6 - 10 | 2 (25) | 41 (44.6) | |
| 11 - 15 | 5 (62.5) | 17 (18.5) | |
a Values are expressed as No. (%).
Among pharyngitis patients, 83.3% of culture-positive cases had oral fever > 38°C, compared to 98.8% of culture-negative cases (P = 0.002). The frequency of anterior cervical adenopathy was higher in culture-positive patients (77.8%) than in culture-negative (47.6%, P = 0.04). No significant differences were found between culture-positive and culture-negative cases regarding headache, cough, gastrointestinal symptoms, pharyngeal erythema, or tonsillar swelling (P > 0.05, Table 3). In summary, GAS was isolated in nearly one-fifth of symptomatic cases and 8% of asymptomatic children, confirming the role of asymptomatic carriers in the epidemiology of pharyngitis.
| Clinical Signs | Culture | P-Value | |
|---|---|---|---|
| + | - | ||
| Fever | 83.3 | 98.8 | 0.002 |
| Anterior cervical adenopathy | 77.8 | 47.6 | 0.04 |
| Sore throat | 100 | 93.9 | 0.28 |
| Headache | 72.2 | 65.9 | 0.60 |
| Cough | 22.2 | 34.1 | 0.32 |
| Runny nose | 5.6 | 24.4 | 0.07 |
| Tonsil swelling | 88.9 | 84.1 | 0.61 |
| Pharyngeal erythema | 94.4 | 96.3 | 0.71 |
a Values are expressed as percentage.
5. Discussion
The GAS remains the most common bacterial cause of acute pharyngitis in children, responsible for 20 - 30% of sore throat cases (15). In our study, 18% of children with pharyngitis and 8% of healthy controls had positive GAS cultures. In a study conducted by Tesfaw et al. in the health centers of Jimma city, the prevalence of group A Streptococcus in pharyngeal cultures of children aged 5 to 15 years with pharyngitis was 11.3% (16). In Turkey, it was 11%, in Brazil, 12%, and among healthy students in Ethiopia, it was 9.7% (17-19). In India (2.8%), Taiwan (4.1%) and Indonesia (7.9%), the prevalence of group A Streptococci was much lower than in our study (20-22). On the other hand, the prevalence of 18% group A Streptococcus in our study was much lower than the 40.6% reported in Ethiopia (23) and 41.5% in Yemen (24). The difference in the prevalence of GAS in different studies may be due to the difference in the seasons when the samples were collected and the studies were conducted. Because in some seasons, the amount of GAS contamination may reach the maximum. In addition, such a difference may be due to differences in methodology and geographical diversity of the studied environments.
In our study, 83.3% of patients with culture-positive pharyngitis had oral fever above 38°C, while 98.8% of patients with culture-negative pharyngitis had fever. This difference was statistically significant. The frequency of anterior cervical adenopathy in patients with positive culture was significantly higher than in patients with negative culture. The frequency of headache, gastrointestinal symptoms, runny nose, cough, pharyngeal exudate, pharyngeal erythema, palatal petechiae, painful adenopathy, and tonsil swelling in patients with positive and negative pharyngeal cultures did not have a significant difference. In Tesfaw et al. study, absence of cough, presence of tonsil swelling or exudate, and body temperature > 38 degrees (P < 0.05) were found as independent predictors for GAS infection in children with pharyngitis (16).
In the study of Sharifian et al., fever, purulent exudate of the pharynx and tonsils, and adenopathy were more common in culture-positive patients than in culture-negative patients (25). In the study by Karami et al., conducted in Zanjan hospitals, fever was higher in positive culture patients than in negative culture patients. However, this difference was not significant (26). However, it is significant to know that the clinical variables that are predictive of GAS infection may differ according to different GAS strains, geographic region, and immune profile of the study population.
In the present study, the highest rate of Streptococcus throat culture positivity was observed in patients with pharyngitis in the age group of 6 - 10 years. In the Ba‐Saddik et al. study, the highest prevalence of GAS pharyngotonsillitis was in the 11 - 15 years old and the age when children were transferred from primary to secondary classes with more crowding and were potentially more exposed to GAS (24). According to Ba‐Saddik et al., children in this age group may not show typical symptoms of GAS pharyngotonsillitis, which makes the diagnosis difficult and emphasizes the importance of laboratory confirmation. The GAS pharyngotonsillitis peaked in the cool months of November, December, January, and February, which are common in other Northern Hemisphere countries (24). In our study, the highest rate of pharyngeal culture positivity for group A Streptococcus was observed in patients with pharyngitis in autumn and in healthy people in winter. In a similar study, the seasonal prevalence of the exudative pharyngitis in the children aged 3 to 15 years was as winter, autumn, spring, and summer, respectively (27).
Differences in prevalence across studies can be attributed to seasonal variation, regional climatic differences, sampling periods, and diagnostic techniques. Interestingly, a higher fever rate was observed among culture-negative patients (98.8%) than in culture-positive patients (83.3%). This unexpected pattern may reflect the presence of viral etiologies among culture-negative cases, as viral infections such as adenovirus or influenza commonly cause higher fevers. Another explanation could be that some bacterial infections were partially treated or that GAS-positive patients were sampled early, before full systemic symptoms developed. This highlights the limitation of relying solely on fever to distinguish bacterial from viral pharyngitis. The frequency of anterior cervical adenopathy was significantly higher among culture-positive children, consistent with other studies that identified adenopathy and tonsillar exudate as predictors of GAS infection (25, 26). However, variables such as headache, cough, and gastrointestinal symptoms were not significantly associated with culture positivity, similar to findings from previous regional studies.
The age group of 6 - 10 years showed the highest prevalence of GAS infection, supporting the concept that school-aged children are most exposed due to crowded environments and close contact. Seasonal clustering in autumn and winter suggests that cooler temperatures and indoor crowding facilitate GAS transmission. The presence of 8% positive GAS cultures among healthy controls indicates asymptomatic carriage, a phenomenon reported in 5 - 20% of children globally. These carriers may serve as reservoirs for bacterial transmission, contributing to overdiagnosis when only clinical features are used. Therefore, laboratory confirmation remains crucial before prescribing antibiotics, particularly in low-resource settings. The study’s findings have important implications for antibiotic stewardship in pediatric care. Overdiagnosis of streptococcal pharyngitis leads to unnecessary antibiotic prescriptions, which in turn promote antimicrobial resistance (AMR) and increase health care costs.
Implementing simple diagnostic algorithms and improving access to RADTs can reduce inappropriate antibiotic use and ensure that only true bacterial infections are treated. Our results emphasize the need for public health education to raise awareness among physicians and parents about rational antibiotic use, particularly in developing regions. These findings can help inform national guidelines for managing pediatric sore throat and reduce the burden of AMR. This study was limited by its single-center design and small sample size, which may restrict generalizability. The lack of molecular confirmation (e.g., PCR) and antibiotic susceptibility testing also limits the diagnostic depth. Future multicenter studies with larger and more diverse populations are recommended to validate our findings and explore molecular epidemiology trends.
5.1. Conclusions
The prevalence of GAS pharyngitis in children aged 2 - 15 years was 18%. The detection of GAS in healthy controls confirms the presence of asymptomatic carriers, emphasizing the need for culture or RADT confirmation before antibiotic prescription. Clinical overdiagnosis and irrational antibiotic use remain public health challenges. Promoting diagnostic accuracy and stewardship programs will be essential to prevent complications and limit AMR.