1. Background
Toxoplasmosis is a zoonotic parasitic disease caused by the intracellular protozoan Toxoplasma gondii, which poses a significant global public health challenge (1). This parasite exhibits a broad host range, infecting various warm-blooded animals, including humans, and has a worldwide distribution (2). Serological evidence reveals that T. gondii exposure is widespread, with hundreds of millions of people around the world carrying specific antibodies, underscoring the parasite’s extensive and pervasive distribution across diverse geographic and demographic landscapes (3). Humans can acquire toxoplasmosis through multiple pathways, such as ingestion of contaminated food, water, or soil containing oocysts, as well as consumption of undercooked or raw meat harboring tissue cysts. Another important transmission route is vertical transmission, where an infected mother transmits the parasite to her fetus, particularly when the primary infection occurs during pregnancy.
This vertical transmission can result in congenital toxoplasmosis, a condition in which the parasite crosses the placenta and invades fetal tissues, leading to serious fetal manifestations such as neurological impairment, hydrocephalus, intracranial calcifications, chorioretinitis, and permanent vision loss in the newborn (4, 5). The diagnosis of congenital toxoplasmosis primarily relies on the detection of T. gondii-specific immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies in the serum of pregnant women. However, accurately determining the timing of maternal infection remains challenging, as distinguishing between acute and chronic infections is critical for effective clinical management and intervention. The persistence of IgM antibodies for extended periods and their potential cross-reactivity with other factors, such as rheumatoid factor and anti-nuclear antibodies, can complicate the interpretation of serological results (6-9).
To address these diagnostic challenges, IgG-avidity testing has been established as a valuable serological tool for the diagnosis of congenital toxoplasmosis. This assay assesses the binding strength (avidity) of IgG antibodies to T. gondii antigens, which is typically low during the acute phase of infection and increases as the infection matures. Thus, IgG-avidity testing aids in differentiating recent from past infections, facilitating more accurate risk assessment and management in pregnant women (10). In Iran, the seroprevalence of toxoplasmosis demonstrates considerable regional variation, influenced by factors such as geography, local dietary practices, and cat ownership (11). Previous studies indicated that seroprevalence rates vary widely, ranging from as low as 10% in southeastern provinces (12) to as high as 70% in northern regions (13), with an overall estimated prevalence of 41% among pregnant women nationwide (14). These disparities underscore the need for region-specific epidemiological studies to inform targeted public health interventions. Maternal serological screening is a crucial preventive strategy for congenital toxoplasmosis.
2. Objectives
Understanding the epidemiology of toxoplasmosis among pregnant women is vital for informing public health initiatives aimed at reducing the incidence of this infection and its associated risks. Consequently, these measures contribute to a decline in the occurrence of congenital toxoplasmosis. Therefore, the present study was aimed to evaluate the prevalence of T. gondii infection among pregnant women attending healthcare centers of Jiroft and to assess the associated risk factors within this vulnerable population.
3. Methods
3.1. Study Area
Jiroft city (28°40′47″N 57°44′41″E) is located in southern Kerman province, Iran, within the Halil River valley and bordered by the Zagros Mountains and Lut Desert. With a population of about 135,000, Jiroft is known for its fertile plains and archaeological significance. The region experiences hot, dry summers, with temperatures often exceeding 45°C (15).
3.2. Study Population
In this cross-sectional survey, the study population comprised pregnant women who were referred to healthcare centers, affiliated with Jiroft University of Medical Sciences, between March 2023 and September 2024. The optimal sample size was determined using the formula:
Where n is the required sample size, p = 0.41 (expected prevalence of T. gondii infection in Iran), d = 0.05 (desired margin of error), and z = 1.96 (for a 95% confidence level), yielding a minimum of 372 participants. To account for potential non-responses or sample attrition, a total of 400 pregnant women were ultimately enrolled in the study.
3.3. Questionnaire
A structured questionnaire was administered to each participant to collect demographic and relevant exposure information, including age, place of residence, education level, gestational age, history of abortion, close contact with cats, close contact with soil, and methods of vegetable washing.
3.4. Serological Tests
Two mL of blood were collected from each participant and transported to the central laboratory of Jiroft University of Medical Sciences. The samples were centrifuged at 800 g for five minutes to separate the serum, which was then stored at -20°C until analysis. The presence of anti-Toxoplasma IgM and IgG antibodies was assessed using enzyme-linked immunosorbent assay kits (Torch-IgG, IgM-Trinity Biotech Company), following the manufacturer’s instructions. Samples with international units (IU)/mL of < 0.9, 0.9 - 1.1, and > 1.1 were classified as negative, borderline, and positive, respectively (16). Subsequently, IgG avidity testing was performed on samples positive for IgG antibodies using indirect enzyme-linked immunosorbent assay with a commercial kit (Euroimmun, Germany), following the manufacturer’s protocols.
The Relative Avidity Index (%) was calculated by dividing the optical density (extinction) of the urea-treated sample by that of the untreated sample. A Relative Avidity Index of < 40% was interpreted as low avidity, > 60% as high avidity, and 40 - 60% as borderline (17). According to the manufacturer’s instructions, serum samples were diluted at a ratio of 1:100. Following completion of the enzyme-linked immunosorbent assay procedures, the optical density values of blanks, controls, and samples were measured using an enzyme-linked immunosorbent assay reader (Biotek ELX 800TS, USA) at a wavelength of 450 nm (18).
3.5. Statistical Analysis
Descriptive statistics, including measures of central tendency and dispersion (mean and standard deviation), as well as relative frequencies, were used to summarize the data. Univariate and multiple logistic regressions were performed to assess the associations between toxoplasmosis infection — defined by IgG seropositivity — and various demographic and individual characteristics. All statistical analyses were conducted using SPSS software (version 25; SPSS Inc., Chicago, IL, USA), with statistical significance set at P < 0.05.
4. Results
4.1. General Characteristics of the Participants
Over the study period, a total of 400 serum samples were collected from pregnant women attending healthcare centers in Jiroft. The majority of participants (83%) resided in urban areas, and 81.2% had attained university-level education. The mean age of the participants was 29.5± 3.9 years, with 17.5% experiencing their first trimester of pregnancy, and 3% reporting a history of abortion.
4.2. Seroprevalence and Associated Risk Factors of Toxoplasma gondii
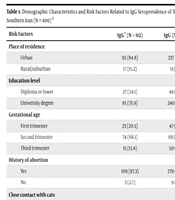
Serological analysis revealed that 112 women (28%) tested positive for Toxoplasma-specific IgG antibodies. Of these, 108 women (27%) tested positive for Toxoplasma-specific IgG antibodies, while an additional four women (1%) exhibited both positive IgG and borderline IgM titers (Table 1). The borderline IgM results in these four cases were consistently confirmed upon repeat testing. Furthermore, IgG-avidity testing demonstrated high avidity in all 112 IgG-positive samples, indicating prior exposure to T. gondii before pregnancy. The results of the multivariate regression analysis assessing the association between potential risk factors and the prevalence of T. gondii IgG antibodies among pregnant women are summarized in Table 1.
| Risk Factors | IgG+ (N = 112) | IgG- (N = 288) | Total (N = 400) | OR | 95% CI | P-Value |
|---|---|---|---|---|---|---|
| Place of residence | 0.77 | |||||
| Urban | 95 (84.8) | 237 (82.3) | 332 (83) | 1 | - | |
| Rural/suburban | 17 (15.2) | 51 (17.7) | 68 (17) | 0.91 | 0.49 - 1.69 | |
| Education level | 0.11 | |||||
| Diploma or lower | 27 (24.1) | 48 (16.7) | 75 (18.8) | 1 | - | |
| University degree | 85 (75.9) | 240 (83.3) | 325 (81.2) | 0.62 | 0.35 - 1.12 | |
| Gestational age | 0.69 | |||||
| First trimester | 23 (20.5) | 47 (16.3) | 70 (17.5) | 1 | ||
| Second trimester | 74 (66.1) | 191 (66.3) | 265 (66.3) | 0.87 | 0.48 - 1.60 | |
| Third trimester | 15 (13.4) | 50 (17.4) | 65 (16.3) | 0.70 | 0.31 - 1.57 | |
| History of abortion | 0.46 | |||||
| Yes | 109 (97.3) | 279 (96.9) | 388 (97) | 1 | - | |
| No | 3 (2.7) | 9 (3.1) | 12 (3) | 0.59 | 0.15 - 2.35 | |
| Close contact with cats | 0.03 b | |||||
| Yes | 16 (42.1) | 22 (57.9) | 38 (9.5) | 1 | - | |
| No | 96 (26.5) | 266 (73.5) | 362 (90.5) | 0.46 | 0.23 - 0.93 | |
| Close contact with soil | 0.26 | |||||
| Yes | 14 (23) | 47 (77) | 61 (15.3) | 1 | - | |
| No | 98 (28.9) | 241 (77.1) | 339 (84.7) | 0.64 | 0.33 - 1.33 | |
| Method of vegetable washing | 0.70 | |||||
| Tap water | 17 (31.5) | 37 (68.5) | 54 (13.5) | 1 | - | |
| Salt solution | 78 (26.6) | 215 (73.4) | 293 (73.3) | 0.75 | 0.39 - 1.42 | |
| Dishwashing liquid | 9 (31) | 20 (69) | 29 (7.2) | 0.95 | 0.35 - 2.57 | |
| Disinfectant solution | 8 (33.3) | 16 (66.7) | 24 (6.0) | 1.10 | 0.38 - 3.21 |
Abbreviations: IgG, immunoglobulin G; OR, odds ratio; CI, confidence interval.
a Values are expressed as No. (%).
b P-value < 0.05.
Among the variables examined, close contact with cats was significantly associated with increased seroprevalence of toxoplasmosis (P = 0.02). Specifically, individuals without close contact with cats had nearly a twofold lower risk of infection compared to those with such contact [odds ratio (OR) = 0.46; 95% confidence interval (CI): 0.23 - 0.93]. The multivariate regression analysis further demonstrated no significant associations between T. gondii IgG seroprevalence and other evaluated risk factors, including residential location, university-level education, gestational age, history of abortion, close contact with soil, and vegetable-washing practices (P > 0.05).
5. Discussion
This study assessed the seroprevalence of T. gondii infection among pregnant women attending healthcare centers in Jiroft, southern Iran, and found an overall prevalence of 28%. This finding aligns with several previous investigations from different parts of Iran, although the reported rates vary substantially across regions (14). Studies from southeastern provinces such as Kerman and Sistan-Baluchistan have reported comparatively lower prevalence rates ranging from 10% to 14% (19, 20), whereas much higher values — up to 40 - 70% — have been documented in the northern provinces of Golestan and Mazandaran (13, 21). These regional variations may be attributed to differences in environmental conditions that affect oocyst survival, dietary behaviors involving consumption of undercooked meat, and exposure to cats or contaminated soil.
A previous investigation in Jiroft reported an IgG seroprevalence of 16.1% among pregnant women (21). The higher rate obtained in the current study may reflect increased exposure to T. gondii in recent years, potentially associated with lifestyle changes such as the growing trend of domestic cat ownership, which raises the risk of environmental contamination with oocysts. Moreover, variations in participant characteristics, sample size, and improvements in assay sensitivity could also explain the observed difference.
The seroprevalence rate observed in this study is comparable to those reported in neighboring countries of the Middle East and North Africa. For instance, prevalence rates of 32 - 44% have been reported in Iraq (22, 23), 30 - 48% in Turkey (24, 25), and 13 - 40% in Saudi Arabia (23, 26). Higher rates exceeding 50% have been documented in Egypt and Sudan (27, 28), whereas lower values are observed in European countries (29, 30), where improved food hygiene and public awareness contribute to reduced transmission. Overall, these comparisons suggest that the seroprevalence in Jiroft represents an intermediate level consistent with patterns reported across the region, reflecting common environmental, cultural, and behavioral determinants of T. gondii exposure.
The finding that 72% of pregnant women were seronegative highlights a substantial proportion of the population susceptible to primary T. gondii infection during pregnancy. Given the risk of severe fetal complications associated with congenital toxoplasmosis, this underscores the urgent need for effective prenatal screening and targeted public health interventions in the region.
Importantly, the use of IgG-avidity testing in this study provided a crucial diagnostic distinction. All 112 IgG-positive samples, including four cases that were borderline for IgM on two consecutive tests, demonstrated high avidity, indicating that exposure to T. gondii occurred prior to the current pregnancy. The persistence of borderline IgM alongside IgG positivity is a recognized phenomenon, as IgM antibodies may remain detectable for months or even years after primary infection and borderline values can result from assay variability or residual antibody activity. The combination of repeated borderline IgM and high IgG avidity is interpreted as evidence of chronic, non-recent infection rather than acute seroconversion. This finding suggests that none of the seropositive pregnant women had acquired a recent or acute infection during pregnancy, which is the period of greatest concern for vertical transmission and congenital toxoplasmosis.
The absence of low or borderline avidity results reinforces the value of incorporating IgG-avidity assays into routine serological screening protocols for pregnant women. By confirming that all seropositive cases represented past infections, the study alleviates immediate concerns regarding the risk of congenital transmission in this cohort and demonstrates that IgG-avidity testing can effectively differentiate between remote and recent infections, thereby guiding appropriate clinical follow-up and counseling.
Among the risk factors evaluated, close contact with cats was the only variable significantly associated with seropositivity. This finding is consistent with the established role of felids as definitive hosts for T. gondii in Iran (31-34) and the world (35-38), and reinforces the importance of educational efforts aimed at reducing high-risk behaviors during pregnancy. Other potential risk factors, including residential location, education level, gestational age, history of abortion, soil contact, and vegetable washing practices, did not show significant associations, which may reflect the high level of education among participants or regional differences in exposure routes.
This study has several limitations that should be considered when interpreting the findings and comparing them with results from other investigations. The absence of molecular confirmatory techniques for acute T. gondii infection may have affected diagnostic accuracy, as serological markers alone can yield ambiguous interpretations in some cases. Moreover, potential information bias arising from incomplete or inaccurate self-reported data on risk factors and exposure history could have influenced the observed associations between participant characteristics and toxoplasmosis seroprevalence. Future studies are encouraged to integrate molecular diagnostics and apply more rigorous data collection approaches to strengthen the validity and reliability of findings.
The findings of this study may not fully represent the broader population of pregnant women in Iran, as toxoplasmosis prevalence and risk factors vary considerably across regions. Differences in climate, cultural practices, and demographic characteristics influence exposure risk, leading to geographic heterogeneity in infection rates. Therefore, caution should be exercised when extrapolating these results to areas with distinct environmental and socio-cultural contexts.
5.1. Conclusions
This study demonstrates a moderate seroprevalence of T. gondii infection among pregnant women in Jiroft, with a large proportion remaining susceptible to primary infection. Close contact with cats emerged as a significant risk factor, underscoring the need for targeted educational and preventive measures. The use of IgG-avidity testing alongside standard serological screening enhances diagnostic accuracy and informs clinical decision-making. These findings advocate for the implementation of comprehensive prenatal screening programs and public health initiatives aimed at reducing the incidence of congenital toxoplasmosis in this region.