1. Background
The prevalence of polycystic ovary syndrome (PCOS) varies among ethnic and racial groups. The PCOS is a public health concern, with a prevalence of about 6 to 21% of premenopausal and reproductive age women (1).
This disease is a genetic and multifactorial environmental disorder that affects the ovaries (2). In PCOS women, obesity, triglyceride (TG) accumulation, insulin resistance, and the risk of developing type 2 diabetes and cardiovascular diseases are increased (3).
The role of sex steroid hormones is important and in three-quarters of patients with PCOS, clinical symptoms, hyperandrogenism, anovulation, dysmenorrhea, oligomenorrhea, and amenorrhea are observed (4).
The syndrome is characterized by a disruption of the hypothalamic-pituitary-gonadal (HPG) axis, which leads to several key hormonal and reproductive dysfunctions (5).
The PCOS may be hereditary, CYP19A1 gene, located on chromosome 15q21.1, codes for aromatase of cytochrome P450 family 19, subfamily a Cyp19a1 (6). The CYP19A1 gene (aromatase: Converts androgens to estrogen) is inhibited in PCOS granulosa cells, leading to abnormal estrogen levels (7).
Granulosa cells express aromatase, which plays an important role in multiple signaling pathways (8). During folliculogenesis, estrogen plays a crucial role in follicular development and granulosa cell dynamics. Changes in aromatase gene expression have also been observed in many diseases (9). A decrease in aromatase activity in ovarian follicles causes an increase in androgens and ovarian failure.
Experimental models offer promising insights, and rodents are affordable, cheap, and easy to obtain, with short estrous and reproductive cycles (10).
Letrozole, (a non-steroidal aromatase inhibitor) a type of aromatase inhibitor, plays distinct roles in humans and mice, primarily related to estrogen production and its impact on various conditions. In humans, this drug plays a role in reducing estrogen levels and slowing or stopping the growth of cancer cells. In mice, letrozole is used as to induce conditions similar to PCOS in mice, allowing researchers to study the effects of androgen excess (11). It disrupts the estrous cycle and an abnormal follicular cycle occurs in ovarian cells (12).
The use of herbal alternative medicines whose physiological effects are known on female reproduction has increased in the past years (13). Traditional medicine in PCOS can influence the treatment of individual symptoms (14).
Rosemary is a small green shrub with pale blue flowers that bloom in late winter and early spring. Rosmarinus officinalis L., a native of the Mediterranean, is an aromatic plant belonging to the Lamiaceae family in food processing as a flavoring and spice (15).
Due to the presence of rosmarinic acid and bioactive phytochemicals, diterpenes, flavonoids, phenolic compounds, and triterpenes such as ursolic acid, the effectiveness of Rosemary extract as an antioxidant, anti-inflammatory, and cosmetic has been commercialized in the treatment and prevention of many diseases (16).
Plants similar to this family (Lamiaceae) Salvia officinalis L. have been used since ancient times to increase fertility in women, eliminate sexual impotence, and treat menopause and menstrual problems, and many female diseases (17).
2. Objectives
The impact of Lamiaceae on women's reproductive health remains largely unknown. This study aimed to investigate the potential protective properties of Rosemary hydroalcoholic extract in PCOS and related reproductive characteristics in a female mouse model induced by letrozole.
3. Methods
3.1. Animal Model
This study was conducted on 30 female NMRI mice following a standard animal care protocol for an experimental study. The details are as follows: Weight: 30 ± 5 grams, source: Royan Institute in Tehran, Iran, temperature: 20 - 25°C, relative humidity: 40%, light cycle: 12-hour light/dark cycle, air-conditioned room, water: Free access to drinking water, diet: Standard pelleted diet food. The protocol adheres to general guidelines for the use and care of living animals in scientific investigations (Council of European Communities 2010) (18).
3.2. Experimental Design
Mice that were regular in terms of the sexual cycle were selected to continue the work and were randomly grouped. Letrozole 1 mg/kg tablet (Aburaihan Pharmaceutical) was used to induce PCOS (each tablet contains 2.5 mg letrozole). Mice were treated with letrozole tablets orally by gavage for 30 days to create PCOS. Rosemary hydroalcoholic extract was prepared by the percolation method, and the desired doses of 50 mg/kg, 100 mg/kg, and 200 mg/kg were used as treatment (19). There were six mice in each group.
1. Group 1: Received sterile distilled water 10 ml/kg body weight for 30 days (control group).
2. Group 2: Letrozole treated, received 1 mg/kg of letrozole 30 days (Sigma-Aldrich, St Louis, MI; model or letrozol group, PCOS) (20).
3. Group 3: The PCOS + Rosemary received a single dose of Rosemary (50 mg/kg, 100 mg/kg, and 200 mg/kg) for 30 days (dose 1, 2, and 3 groups or Let+Ros 50, 100, and 200 mg/kg)
3.3. Determination of Estrus Cycle
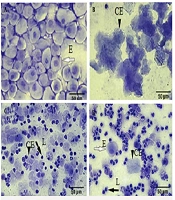
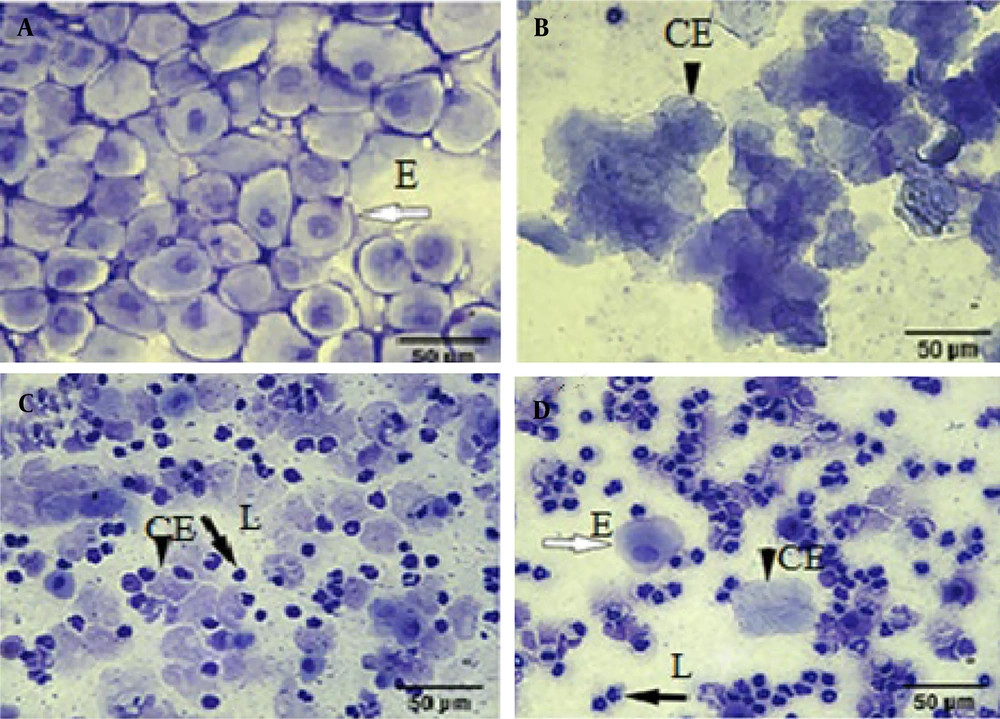
Regular estrous cycles were determined for each mouse via vaginal smear. The stages of the estrous cycle were determined according to the ratio and morphology of leukocytes and epithelial cells. In the proestrus stage, the predominance is with nucleated epithelial cells, in the estrus stage, cornified epithelial cells without a nucleus, and during the next stage meta-estrus stage, the same percentage of cornified epithelial cells and leukocytes. In the diestrus stage, the predominance is also epithelial cells and leukocytes (Figures 1A - D) (21).
3.4. Biochemical Studies
One mL of blood sample was collected from the inferior vena cava and serum was stored at -20°C for hormonal assay. Estrogen concentrations were measured using the Monobind ELISA kit according to the manufacturer's protocol.
3.5. Reverse Transcription and Real-time PCR
According to the study by Naeimi et al., mRNA expression was examined using RT-qPCR (22). The qRT-PCR was performed at 95°C for 10 minutes, followed by 45 cycles of 95°C for 30 seconds, 60°C for 45 seconds, and 72°C for 30 seconds. The relative quantity of mRNA was evaluated by the 2-ΔΔCT technique. Table 1 shows the sequences of the primers designed by the OLIGO primer analysis software (version 7.0).
| Genes | Primer Sequences | TM |
|---|---|---|
| Forward-GAPDH | AGGTCGGTGTGAACGGATTTG | 61 |
| Reverse-GAPDH | TGTAGACCATGTAGTTGAGGTCA | 61 |
| Forward-Cyp19a1 | CTGGCCGGAGGTCTTTGTTC | 60.96 |
| Reverse-Cyp19a1 | GCATGGGGTTCAGCATTTCC | 59.82 |
3.6. Histopathological Analysis
Ovaries from experimental and control mice were stored in PBS buffer, then fixed in Bouin's solution for 24 h for morphological examination. After preparation, 5 μm thick sections were prepared and stained for histopathological examination, and then the number of follicles in each ovary at different stages of development was counted (Olympus CX31 microscope; Olympus, Tokyo, Japan).
3.7. Total Triglyceride Analysis
To assess TG levels, blood serum stored at -20°C was used, which was measured using animal TG kits for biochemical analysis of total fat by standard methods.
3.8. Statistical Analysis
Statistical analyses were performed with SPSS 22 software. We used GraphPad Prism version 5 to determine significant differences between treatment and control groups (mean ± SEM) and one-way ANOVA followed by multi-Tukey (normal data distribution). P < 0.05 was considered statistically significant.
4. Results
4.1. Estrous Cycle
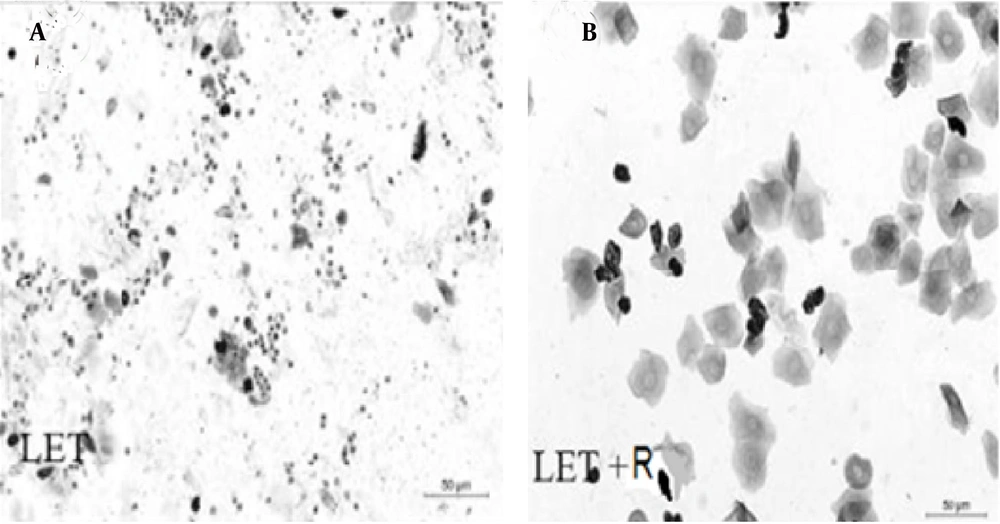
Comparison of vaginal smears in the control and letrozole groups showed that in letrozole group only leucocytes, as the dominant cell type of the diestrus phase. Therefore, in this group (Figure 2A) the prolongation of the diestrus phase confirmed the irregular estrous cycles and letrozole-induced mice exhibited a prolonged diestrus cycle. In the Rosemary groups, epithelial nucleated cells or cornified cells confirmed the restoration of a regular estrous cycle, similar to that in control mice (Figure 2B).
4.2. Hormonal Analysis
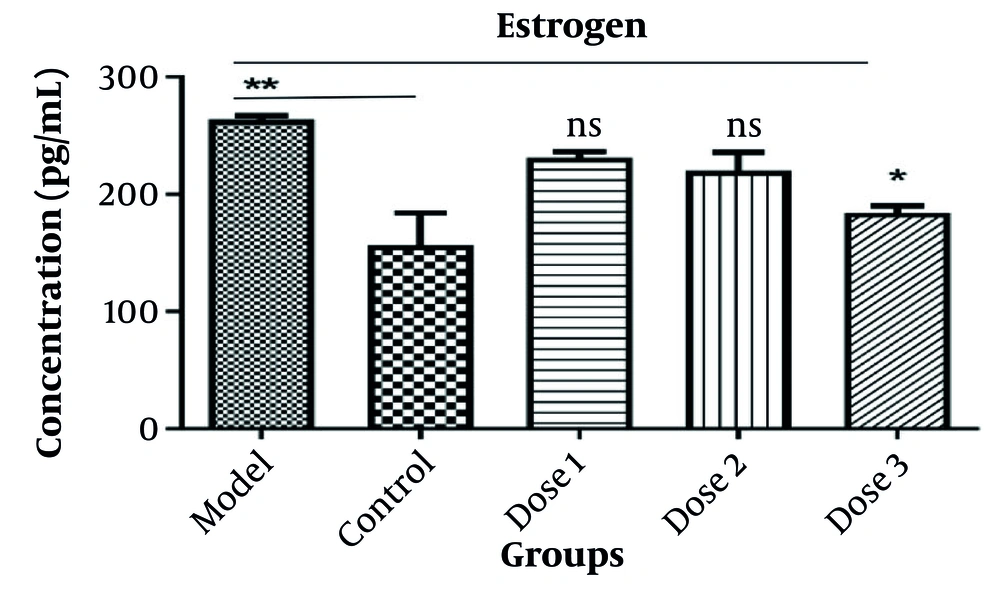
Serum estrogen levels increased in letrozole group compared to the control group, and this relationship was significant (P = 0.099). The hormone level in 3 doses of Let+Ros 50, 100, 200 mg/kg groups was decreased compared to the model group (letrozole group).
Estrogen was improved at Let+Ros 50, 100, 200 mg/kg, especially the dose (200 mg/kg; P = 0.03). Hormone levels increased in the letrozole group compared to the control group (72%) and decreased in dose 3 (35%) compared to the model group (Figure 3).
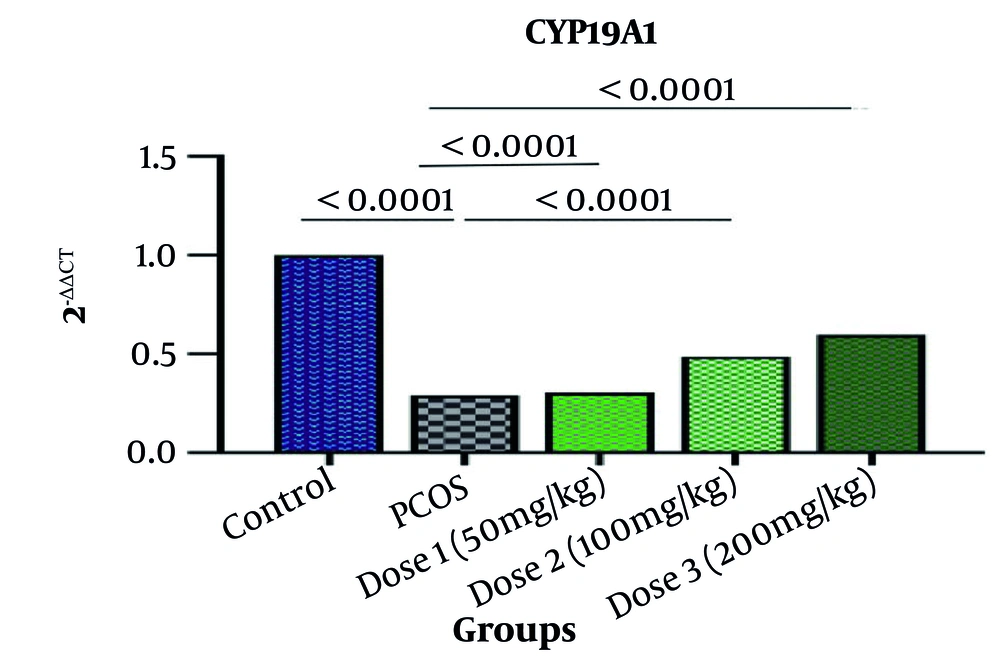
4.3. CYP19A1 Gene Expression
CYP19A1 gene expression was significantly reduced in the letrozole group compared to the control group (P = 0.0999). In the experimental groups treated with the Rosemary extract compared to letrozole group (PCOS), depending on the dose used, Let+Ros 50, 100, and 200 mg/kg respectively, the lowest to the highest improvement was achieved (Figure 4). Rosemary treatment increased aromatase gene expression compared to the PCOS group. Mice treated at doses 1, 2, and 3 showed 6%, 31%, and 54% increased expression compared to letrozole group mice, respectively (P < 0.0001).
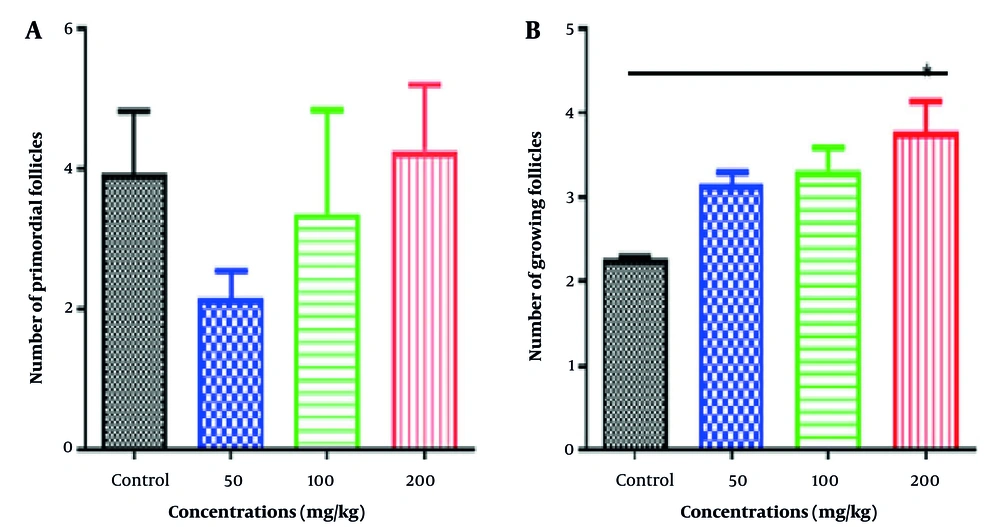
4.4. Change in Follicles Number
To determine follicular morphometric changes, we divided them into six groups based on the morphology and diameter of the follicle types: Primordial follicles, primary follicles, growing follicles, antral, cystic follicles, and corpus luteum. In the Rosemary hydroalcoholic extract groups (Let+Ros 50, 100, and 200 mg/kg) compared to the control group, the number of cysts and their size decreased, and several corpora lutea were also observed in most of them, indicating the onset of ovulation in them. There was also no significant difference in the number of primary follicles, but the number of developing follicles gradually increased, although this increase was only significant at the Rosemary 200 mg/kg (Let+Ros 200; P = 0.035, Figures 5A and B).
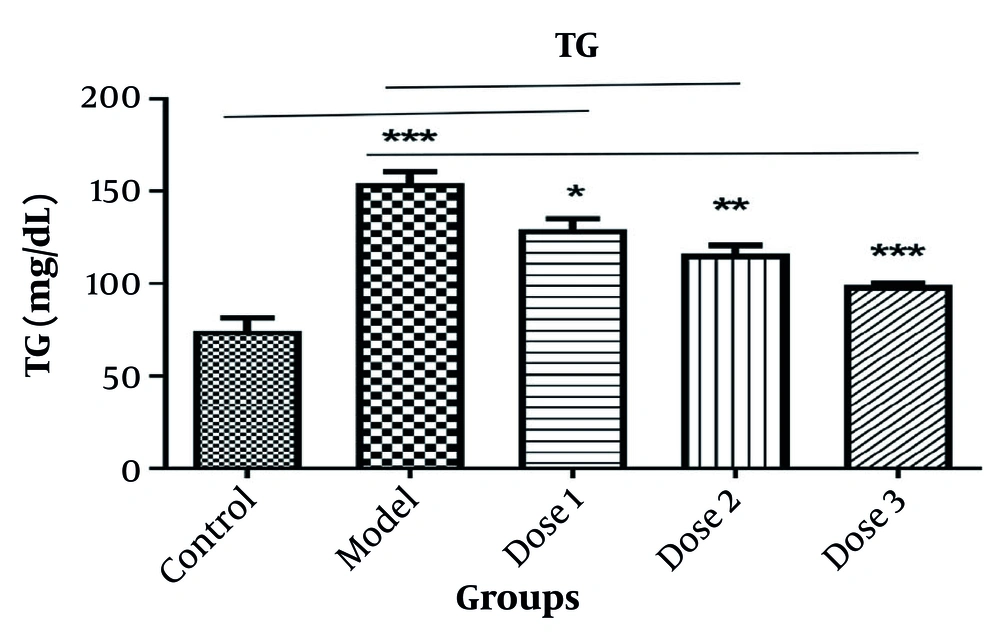
4.5. Triglyceride Assessment
In the PCOS group (letrozole or model group; 150 ± 2.5), the increase in TG was significantly greater (92%) than in the control group (71 ± 2.6; P < 0.0001). The TG levels were significantly reduced in doses 1, 2, and 3 (Let+Ros50, 100, and 200 mg/kg; 125 ± 2.8, P = 0.02; 111 ± 3.2, P = 0.099; and 97 ± 2.1, P = 0.0999) respectively, compared to the letrozole or model group. The greatest reduction was observed in the dose 3 (200 mg/kg) group (Figure 6).
5. Discussion
The PCOS is known as a heterogeneous disease. In this study, the effect of letrozole and R. officinalis on PCOS was investigated. According to Figure 2, in PCOS mice, the estrous cycle was changed and irregular, and then it was improved by treating the mice with Rosemary hydroalcoholic extract at different doses. In the letrozole group, the diestrus phase was prolonged and mice treated with Let+Ros 50, 100, and 200 mg/kg showed nucleated epithelial cells and returned to a regular estrous cycle.
Yang et al. examined the estrous cycle in rats treated with Ecklonia cava extract. This extract was useful for the management of PCOS and suggested a potential treatment for PCOS, which was similar to the results of Rosemary in our study (23).
The our report showed that the Let+Ros 50, 100, 200 mg/kg had beneficial effects on the hormonal changes of mice with PCOS, which causes changes in the estrous cycle, and the number of growing follicles.
In Lee's study, the effect of Allium fistulosum root extract showed a significant difference in epithelial nucleated cells between rats induced with letrozole and letrozole + A. fistulosum extract. Vaginal smear cells showed increased leukocytes and cessation of the estrous cycle in PCOS mice (24).
The results showed that the use of letrozole significantly increased serum estrogen levels in mice with PCOS compared to the control group, and after receiving Let+Ros 50, 100, and 200 mg/kg, estrogen levels showed a significant decrease at dose3.
Rosemary extract can improve ovarian function by changing steroid levels and ovarian steroidogenesis, and may treat female infertility in traditional medicine (25). In 2015, it was reported that hormonal changes in mice with PCOS were reversed by curcumin (26).
According to Figure 3, the increase of estrogen in PCOS mice (model or letrozol group) was significant (P < 0.01) and the hormone level decreased in Let+Ros 50, 100, and 200 mg/kg (P < 0.05).
The results of Naseran's research were consistent with our study, they showed that the use of letrozole to induce PCOS caused a significant increase in estrogen, which decreased after receiving a combination of black seed hydroalcoholic extract and honey (2400 mg/kg). As in our study, the dose of 200mg/kg extracts caused the greatest decrease in the hormone amount (27).
Some studies associated PCOS with changes in aromatase expression and serum oestradiol level. In the PCOS group in the study by Jafari khorchan et al., estrogen levels increased, thereby reducing oogenesis. The results of a 2020 study also showed that quercetin significantly reduces estrogen levels and may be useful in treating fertility problems in PCOS patients (28). Rosemary is a regulator of metabolic and steroid properties that can be considered as a complementary treatment in patients with PCOS with further studies (29).
In another study, contrary to our results, plasma estrogen levels were decreased by letrozole induction, and the low level was restored after Mahuang-Tang (MHT) treatment traditional medicine improves ovarian dysfunction and the regulation of steroidogenic genes in letrozole-induced PCOS rats (30). Also, in study by Kafali et al. showed that plasma hormone levels of letrozole-induced rats increased, such as LH and testosterone, and decreased estrogens (7).
In conditions like PCOS, increased androgen levels can result from both adrenal and ovarian overproduction and peripheral conversion of precursors.
Adrenal androgen synthesis is the process by which androgens produced by the adrenal cortex are converted into other hormones, including male sex hormones (such as testosterone) and female sex hormones (such as estradiol and progesterone). The research by Tait and Hodge concluded that the sesterterpene pathway for steroid biosynthesis can function in the rat and human adrenal glands to produce androgens and that the intermediates are converted to androgens in the microsomal fraction (31).
Increased gene expression will lead to increased production of the corresponding protein. In other words, gene expression is the process by which the information contained in a gene is converted into a functional product, usually a protein. Therefore, the evaluation of Cyp19a1 gene expression reflects a decrease in aromatase protein levels in the letrozol group and an increase in this protein in the Let+Ros 50, 100, and 200 mg/kg.
Our results showed that the improvement in ovarian function in Rosemary extract treatment in mice resulted in increased aromatase activity and increased Cyp19a1 gene expression. Also, Cyp19a1 gene expression increased with increasing Let+Ros 200 mg/kg, which was significant compared to the control and letrozole groups
Consistent with our results, a study by Panghiyangani et al. showed that the relative mRNA expression of CYP19A1 (aromatase) in the PCOS group was 0.38 ± 0.25, while in the non-PCOS group it was 1.00 ± 0.00. Reduced aromatase activity helps increase testosterone levels. This condition contributes to the hyperandrogenism that is a hallmark of women with PCOS (6).
The increase in Cyp19a1 expression by MHT treatment was consistent with the results of our study (30). Previous studies have reported insufficient aromatase activity in PCOS, suggesting that Cyp19a1 plays a key role in the normal progression of the menstrual/estrous cycle in PCOS mice (6).
In a study by Chen et al., the relationship between aromatase activity and obesity in women with and without PCOS showed that low aromatase activity was associated with low levels of Cyp19a1 mRNA expression (32). Down-regulation of CYP19A1 in letrozole-treated rats reduces aromatase expression in granulosa cells (33).
In mice treated with 200 mg/kg of Rosemary, the number of growing follicles during the follicular maturation process was reduced due to the adverse effects of letrozole. In other studies, fewer cysts and growing follicles, and corpora alutea in the ovaries indicated follicular maturation and ovulation, which was consistent with our study (1).
Also, Amanat et al. showed that the use of genistein (20 mg/kg) increases luteinization and less cyst formation in histopathological analysis (P < 0.05) (34). In Yang et al.'s study, the effects of MHT on the ovarian histology of letrozole-induced rats were investigated, and the number of cystic follicles was significantly reduced after MHT treatment (30).
In the comparison of ovaries induced with letrozole and A. fistulosum in rats, the number of follicular cysts in rats induced with letrozole (13.50 - 4.37) was significantly increased compared to the control group (7.83 - 2.64) and in group letrozole, + A. fistulosum (4.43 - 1.13) decreased (24). Also, the data showed that using a higher dose (60 mg/kg) of sage extract, the number of growing follicles increased because estradiol plays a vital role in the maintenance and growth of follicles (35). This study showed that Rosemary therapy leads to a reduction in PCOS symptoms and can be a safe and effective component to improve PCOS complications.
It is hypothesized that Rosemary antioxidant molecules may act as free radical scavengers and play a role by regulating the activity and/or expression of specific enzyme systems involved in relevant physiological processes such as intracellular signal transduction via phenolic phytochemicals. Rosemary extract is able to prevent free radicals from entering biological molecules (36).
The use of antioxidants such as Rosemary extract for their potential protective effects to reduce oxidative stress may have therapeutic benefits for improving women with PCOS (37).
In addition to its antioxidant properties, Rosemary affects the expression of the CYP19A1 gene, and increased aromatase activity causes changes in hyperandrogenism, which is a characteristic of PCOS. The receptor in the membrane of granulosa cells causes the stimulation of promoter II activity by FSH. In the proximal II promoter, aromatase activity is expressed in a way that triggers estrogen secretion from the preovulatory follicle (6).
Other properties of Rosemary that Safavi et al. demonstrated in 2024 include the effect of Rosemary and ketamine gargling on hoarseness and sore throat after endotracheal intubation. The Rosemary group had less sore throat because the Rosemary solution contained carnosol and carnosic acid, which have high potential anti-inflammatory and antioxidant agents that reduce the production of interleukin beta and TNFα, cyclooxygenase-2 (COX-2) enzymes, and prostaglandins. The analgesic effect of these compounds may be due to their antispasmodic and antiprostaglandin effects (38).
5.1. Conclusions
The present study showed that Rosemary hydroalcoholic extract effectively inhibited PCOS symptoms induced by letrozole. Based on the results, we suggest that the components in Rosemary hydroalcoholic extract may act as a potent therapeutic drug for PCOS. Today, due to the adverse effects of chemicals and hormones, plant extracts are important for reducing complications caused by treatment. However, the effects of Rosemary hydroalcoholic extract on ovarian function and its associated mechanisms require further investigation because anovulation and prevention of full egg maturation increase the risk of infertility in women with PCOS. Therefore, studying PCOS-related genes and underlying physiological pathways and the effect of other effective herbal extracts is suggested.