1. Introduction
Sexually transmitted diseases (STDs) constitute a major global public health burden, transmitted through sexual contact and associated behaviors (1). These infections, which include viral agents such as human papillomavirus (HPV) and bacterial pathogens like Treponema pallidum, Neisseria gonorrhoeae, and Chlamydia trachomatis (CT), contribute significantly to morbidity, reproductive health complications, and socioeconomic strain (2, 3). Despite ongoing efforts, prevention and treatment strategies remain suboptimal, underscoring the need for novel molecular insights (4).
Recent advances in genomics and bioinformatics have highlighted the regulatory roles of microRNAs (miRNAs) in infectious diseases. MicroRNAs are small, non-coding RNAs that fine-tune gene expression post-transcriptionally, influencing immune responses, cellular signaling, and pathogen persistence (5-7). In STDs, miRNA dysregulation has been linked to disease progression, immune evasion, and clinical outcomes, presenting opportunities for improved diagnostics and targeted therapies (8, 9). This review aims to consolidate current knowledge on miRNA involvement in both viral and bacterial STDs, with an emphasis on mechanistic pathways and clinical implications, thereby addressing a critical gap in the integrative understanding of miRNA-mediated regulation in STD pathogenesis.
2. Structure and Function of MicroRNAs
MicroRNAs are highly conserved, single-stranded noncoding RNAs, typically 21–25 nucleotides in length, that regulate gene expression post-transcriptionally (10, 11). By binding to the 3′-untranslated region (3′-UTR) of target mRNAs, miRNAs guide the RNA-induced silencing complex (RISC) to degrade transcripts or suppress translation (12, 13). This regulatory capacity allows miRNAs to maintain cellular homeostasis under physiological conditions.
In pathological states, miRNA expression is frequently altered, contributing to diseases such as cancers, inflammatory disorders, and infections (14, 15). Conversely, certain miRNAs may also promote homeostasis, reflecting their dual roles in health and disease. In the context of STDs, miRNAs modulate host immune responses and pathogen-related signaling pathways, influencing infection outcomes (8, 16).
3. Correlation Between MicroRNAs and Sexually Transmitted Diseases
3.1. Role of MicroRNAs in Genital Warts
Genital warts, or condyloma acuminatum (CA), are primarily caused by HPV types 6 and 11 (17). HPV enters basal epithelial cells and replicates during epithelial differentiation, leading to lesion formation (18). Clinically, genital warts are classified into four types: Classic CA, keratotic warts, popular warts, and flat warts, all characterized by high contagiosity and recurrence (19).
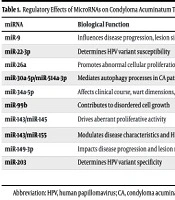
MicroRNAs play a pivotal role in CA pathogenesis by regulating cell proliferation, apoptosis, and differentiation. For example, miR-34a-5p is downregulated in CA tissues, while PD-L1 is upregulated, demonstrating an inverse correlation with diagnostic potential (AUC: 0.954) (20). miR-26a overexpression degrades PTEN mRNA, impairing tumor suppression (21). Loss of miR-143 or miR-145 disrupts NRAS/PI3K/AKT signaling (22), and reduced miR-99b elevates IGF-1R expression, further activating PI3K/AKT and driving proliferation (23). Autophagy-related miRNAs, such as miR-30a-5p and miR-514a-3p, are diminished in CA, while autophagy proteins (Atg5, Atg12, Atg3) are upregulated (24). Additional miRNAs, including miR-22-3p (25), miR-31, miR-9 (26), miR-155, and miR-203 (27), correlate with wart size, HPV subtype, and recurrence, though their precise mechanisms require further study (Table 1).
| miRNA | Biological Function | Molecular Target | Ref |
|---|---|---|---|
| miR-9 | Influences disease progression, lesion size, and HPV strain specificity | HK2 | (28) |
| miR-22-3p | Determines HPV variant susceptibility | VEGF | (29) |
| miR-26a | Promotes abnormal cellular proliferation | PTEN | (30) |
| miR-30a-5p/miR-514a-3p | Mediates autophagy processes in CA pathogenesis | Atg5, Atg12, Atg3 | (31) |
| miR-34a-5p | Affects clinical course, wart dimensions, HPV subtype, and diagnostic potential | PD-L1 | (32) |
| miR-99b | Contributes to disordered cell growth | IGF-1R | (24) |
| miR-143/miR-145 | Drives aberrant proliferative activity | NRAS, PI3K p110α, and phosphorylated AKT | (33) |
| miR-143/miR-155 | Modulates disease characteristics and HPV strain specificity | NRAS, PI3K p110α, and phosphorylated AKT | (34) |
| miR-149-3p | Impacts disease progression and lesion morphology | HE4 | (35) |
| miR-203 | Determines HPV variant specificity | p63 and Survivin | (36) |
Abbreviations: HPV, human papillomavirus; CA, condyloma acuminatum; HK2, hexokinase 2; VEGF, vascular endothelial growth factor; PTEN, phosphatase and tensin homolog; Atg, autophagy-related proteins; PD-L1, programmed death-ligand 1; IGF-1R, insulin-like growth factor 1 receptor; HE4, human epididymis protein 4; MicroRNAs, miRNA, microRNA.
3.2. Role of MicroRNAs in Syphilis
Syphilis, caused by Treponema pallidum, progresses through distinct clinical stages (37). Beyond traditional serological tests (28), miRNAs show promise for early detection and monitoring. miR-338-5p is elevated in latent syphilis and associated with T-cell receptor signaling (38). Differential expression of miR-195-5p, miR-223-3p, and miR-589-3p distinguishes serofast from cured patients (39). Mechanistically, miR-101-3p downregulates TLR2, reducing cytokine production in macrophages (31), while miR-142-3p impairs phagocytosis in dendritic cells and macrophages during secondary syphilis (35) (Table 2).
| miRNA | Biological Function | Molecular Target | Ref |
|---|---|---|---|
| miR-101-3p | Suppresses cytokine production in macrophages | TLR2 3' UTR | (4) |
| miR-142-3p | Enhances syphilis disease progression | DC | (6) |
| miR-195-5p/miR223-3p, mir-598-3p | Serves as diagnostic biomarker and treatment response predictor | Multiple gene targets | (40) |
| miR-338-5p | Identifies serofast patients and aids in latent syphilis diagnosis | RANBP17, XPO1, and XPO6 | (2) |
Abbreviations: TLR2, toll-like receptor 2; UTR, untranslated region; DC, dendritic cells; RANBP17, RAN binding protein 17; XPO1, Exportin 1; XPO6, Exportin 6; miRNA, microRNA.
3.3. Role of MicroRNAs in Gonorrhea
Gonorrhea, caused by Neisseria gonorrhoeae, affects millions annually, with rising antimicrobial resistance (33, 41). Bacterial lipo-oligosaccharide (LOS) induces miR-146a overexpression in monocytes, inhibiting NF-κB via IRAK1/TRAF6 and suppressing TNF-α and IL-1β production, which may facilitate bacterial persistence (42). Reduced miR-718 in infected macrophages enhances PI3K/AKT signaling via PTEN downregulation, dampening inflammatory responses and increasing host susceptibility (43).
3.4. Role of MicroRNAs in Genital Chlamydia trachomatis Infection
The CT infections are often asymptomatic but can lead to pelvic inflammatory disease and infertility (36, 44). Dysregulated miRNAs influence susceptibility and outcomes. miR-146a and miR-155 alter vaginal microbiota and T-cell function (45). Symptomatic infections show elevated miR-142 and miR-147, while asymptomatic cases upregulate miR-449c, miR-6779, and miR-519d (46). Experimentally, miR-378b deficiency impairs bacterial clearance but reduces inflammation (47). miR-30c-5p modulates mitochondrial dynamics via Drp1, inhibiting CT replication (48), and miR-135a regulates CD4+ T-cell trafficking through the CXCL10/CXCR3/CCR5 axis (30) (Table 3).
| miRNA | Biological Function | Molecular Target | Ref |
|---|---|---|---|
| miR-30c-5p | Impairs bacterial growth and alters mitochondrial dynamics | Tumor protein p53 | (19) |
| miR-135a | Modulates immune cell trafficking and regulatory functions | CXCL10 | (23) |
| miR-142/miR-147/miR-449c/miR-6779/miR-519d/miR-449a/miR-2467 | Potential biomarkers for tracking disease progression | Multiple gene targets | (44) |
| miR-146a/miR-155 | Alters vaginal microbial environment | Not specified | (45) |
| miR-378b | Regulates CT replication and influences reproductive complications | EMT markers | (46) |
Abbreviations: CXCL10, C-X-C motif chemokine ligand 10; EMT, epithelial-mesenchymal transition; miRNA, MicroRNA.
4. Discussion
MicroRNAs serve as master regulators of cellular processes, including differentiation, apoptosis, and signaling, and are increasingly recognized for their roles in STD pathogenesis (5, 49). Their ability to modulate immune responses and pathogen persistence underscores their potential as biomarkers and therapeutic targets (50).
In HPV infection, miRNAs such as miR-34a-5p and miR-26a disrupt PI3K/AKT and MAPK signaling, promoting epithelial proliferation and immune evasion (20, 51). Similarly, in gonorrhea, miR-146a and miR-718 fine-tune NF-κB and PI3K/AKT pathways, influencing bacterial survival and host inflammation (42). Syphilis presents a unique challenge with the serofast state, where miRNAs like miR-338-5p and miR-142-3p modulate TLR pathways and phagocytic functions, impairing clearance of Treponema pallidum (35, 38). In chlamydia infection, miRNAs coordinate immune cell recruitment and bacterial containment through mitochondrial regulation and chemokine signaling (30, 48).
A unifying theme across these infections is miRNA-mediated regulation of key pathways such as PI3K/AKT, TLR/NF-κB, and autophagy. These interactions highlight conserved mechanisms of host-pathogen interplay and suggest broad therapeutic potential. However, translational applications require deeper mechanistic insights and validation in larger clinical cohorts (52).
5. Conclusion
This review underscores the central role of miRNAs in regulating immune responses and signaling pathways in STDs. While significant progress has been made in identifying dysregulated miRNAs and their targets, challenges remain in translating these findings into clinical practice. Future research should focus on:
-Elucidating precise mechanistic networks through integrated omics approaches
-Validating miRNA biomarkers in diverse, large-scale clinical populations
-Developing targeted miRNA-based therapies for enhanced STD management
By bridging molecular insights with clinical applications, miRNA research holds promise for advancing diagnostic accuracy, therapeutic precision, and preventive strategies in STD care.