1. Background
The tear trough, referring to the medial one-third of the periorbital hollow, is a common aesthetic concern contributing to a fatigued or aged appearance (1). While often associated with aging, tear trough deformity can also be present in younger individuals due to anatomical factors (2). Non-surgical correction using hyaluronic acid (HA) fillers has gained popularity due to its minimally invasive nature as opposed to surgical options like fat grafting or blepharoplasty (3). However, challenges such as post-injection edema and skin irregularities have been reported, often linked to filler quality or injection technique (4).
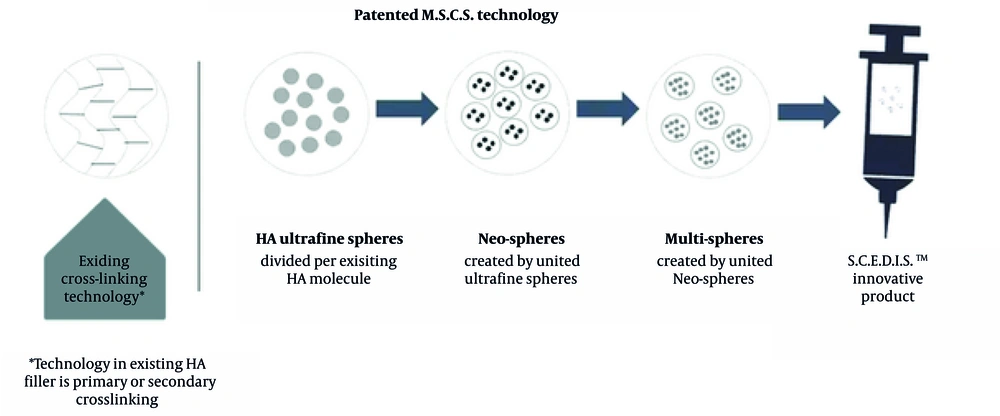
To address these issues, a novel monophasic, mono-densified HA filler, developed using specific cross-linked enhanced dermal integration system-mono-densified sphere creation (SCEDIS-MSCS) technology, was introduced. This technology produces ultrafine HA Neo-Spheres with enhanced intermolecular bonds, improving material stability, tissue integration, and resistance to degradation compared to traditional non-cross-linked or polydensified HA fillers (5). These properties aim to minimize edema and ensure smooth, natural-looking results in the sensitive tear trough area.
2. Objectives
Based on this, the current pilot study explicitly aimed to evaluate the efficacy and safety of the novel HA filler in improving tear trough aesthetics, using both objective Global Aesthetic Improvement Scale (GAIS) and subjective Tear Trough Improvement Scale (TTIS) measures, with plans for a larger randomized controlled trial (RCT) to confirm findings (Figure 1).
3. Methods
3.1. Patients
Sixteen females (age range 30 - 55, 40.5 ± 5.45 years) with obvious tear trough aesthetic deformity were enrolled in the study. All participants had been experiencing an obvious infraorbital hollow for more than 10 years. The exclusion criteria included connective tissue diseases (e.g., lupus erythematosus), blood disorders, and cancer. The use of salicylic acid, vitamin E, Ginkgo Biloba, anti-inflammatory, and anti-platelet drugs was prohibited for 20 days before the application of HA. Patients who had undergone chemical peelings, dermabrasion, fractional laser application, or any type of filler injection in the area under study should have been treated more than 2 years ago. All females provided written informed consent after receiving detailed information about the tear trough filler, and the study was conducted in compliance with Good Clinical Practice and the Declaration of Helsinki, in accordance with Greek law. The study protocol and patient involvement were formally approved by the Local Ethics Committee of Tzaneio General Hospital, Piraeus, Greece.
3.2. Tear Trough Treatment
Each subject was injected with 1 mL of the studied HA filler, approximately 0.5 mL per side. In all cases, a 25 G cannula was used to ensure safer penetration, aiming to avoid puncturing blood vessels and nerves, thereby reducing the risks of bleeding, bruising, and pain (4). Microinjections (0.02 - 0.05 mL/site) were spaced 1 - 2 cm apart, following a standardized protocol.
Outcomes were assessed using: (1) The GAIS, a validated 5-point scale for evaluating aesthetic treatment outcomes (5); (2) high-resolution digital photography (consistent focus, lighting, and file format; participants remained expressionless) taken before and one month post-treatment.
A modified TTIS Questionnaire, adapted from a validated scale (6), administered before, immediately after, and one month post-treatment. The TTIS was designed based on a retrospective analysis of tear trough filler outcomes (7) and was used to assess daily feel-good sense. For that purpose, it was completed at the same time points to capture subjective satisfaction and to monitor side effects like edema.
It should be mentioned that the GAIS score was conducted one month after the tear trough injections since immediate post-injection edema may mislead the objective observation. However, edema, even if short-lasting, is considered a bothersome side effect. Because of this, and since it was of main interest to estimate the daily feel-good sense of the participants, the TTIS Questionnaire was applied before, the first day after, and one month post the injectable aesthetic intervention.
3.2.1. Content of the Modified Tear Trough Improvement Scales and Checklists
3.2.1.1. Tear Trough and Upper Lip Wrinkles Improvement Scale
Please rate your satisfaction with the following aspects of your dermal filler treatment for tear troughs and upper lip wrinkles on a scale of 1 to 5, where: 1 = very dissatisfied, 2 = dissatisfied, 3 = neutral, 4 = satisfied, and 5 = very satisfied.
1. Reduction in the appearance of tear trough deformity.
2. Improvement in the appearance of dark circles or shadows under the eyes.
3. Smoothing of upper lip wrinkles (barcode lines).
4. Enhancement of lip definition and shape.
5. Natural-looking results in the treated areas.
6. Comfort and absence of lumps or irregularities in the treated areas.
7. Harmony of the treated areas with the rest of your facial features.
8. Improvement in the overall youthful appearance of your periorbital and perioral regions.
9. Likelihood of continuing with this dermal filler treatment for long-term maintenance of the results.
10. Likelihood of recommending this dermal filler treatment to others seeking to improve their tear troughs and upper lip wrinkles.
Scoring:
- Add up the scores for all 10 questions.
- Minimum score: 10.
- Maximum score: 50.
- Higher scores indicate greater satisfaction with the dermal filler treatment for tear troughs and upper lip wrinkles.
3.3. Hyaluronic Acid Injectable Fillers
The HA filler, composed of monophasic, mono-densified cross-linked HA chains with enhanced intermolecular bonds, was developed using SCEDIS-MSCS technology to ensure material stability and delayed degradation. As depicted in Figure 2, this technology produces ultrafine HA Neo-Spheres that integrate smoothly into the tear trough, minimizing edema and irregularities compared to traditional polydensified or non-cross-linked HA fillers (5).
3.4. Statistical Analysis
Statistical analysis employed one-way Friedman ANOVA to assess changes across multiple time points, followed by post-hoc Wilcoxon signed-rank tests with Bonferroni correction for pairwise comparisons. The Mann-Whitney U test was used to compare independent groups when applicable. Effect sizes were calculated as percentage changes in means. A significance level of α = 0.10 was chosen to align with the exploratory nature of this pilot study, though future RCTs will adopt α = 0.05. Statistical significance was set at P < 0.10 unless otherwise noted.
4. Results
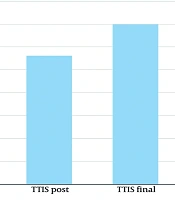
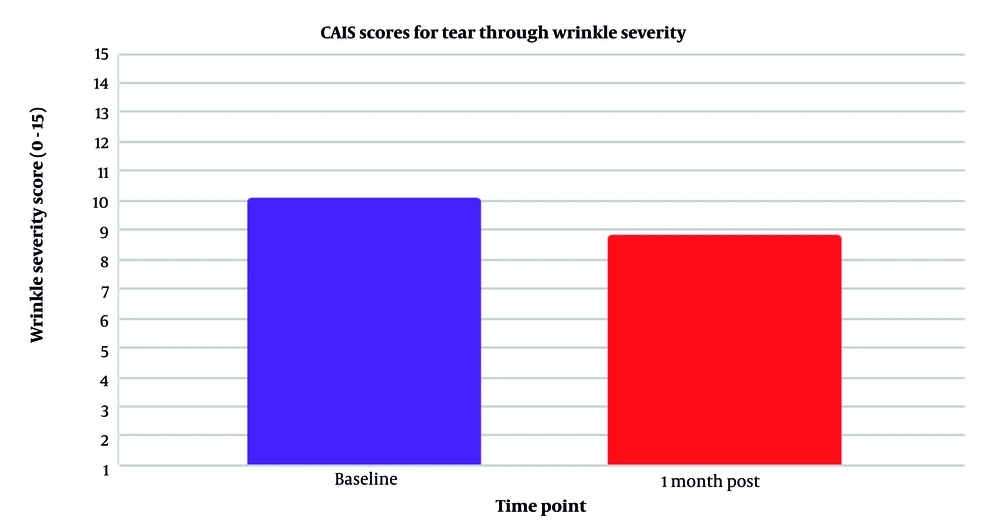
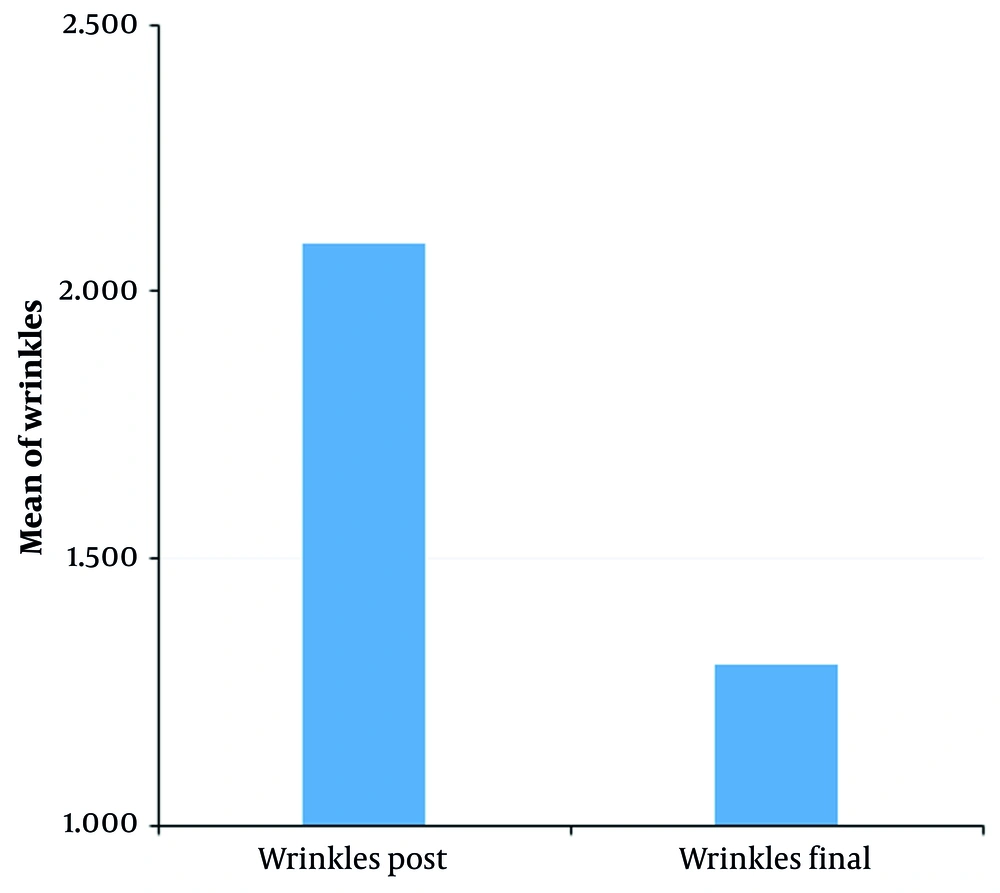
The overall aesthetic refinement of the tear trough area was found to be statistically remarkable. Figure 3, using the GAIS method of wrinkle estimation, depicts the summarized outcome, with the decrease in tear trough wrinkles shown as statistically significant at P = 0.05 (for α = 0.10). Additionally, Figure 1 illustrates an 18.2% reduction in wrinkle severity (P = 0.05, mean ± SD 5.8) according to the GAIS method. Moreover, as clearly shown in Figure 4, the TTIS Questionnaire demonstrated significant improvement in patient satisfaction (P = 0.018 immediately post-treatment, P = 0.001 one-month post-treatment, α = 0.10), with mean scores increasing from 29.2 ± 4.5 to 37.6 ± 4.2 immediately post-treatment (28.8% improvement), and to 41.8 ± 3.9 one-month post-treatment (43.2% improvement; Table 1).
Results of subjective satisfaction feeling [Tear Trough Improvement Scale (TTIS) Questionnaire]; bar charts showing mean satisfaction scores (scale 8 - 40) at baseline, immediately post-treatment (P = 0.018), and one-month post-treatment (P = 0.001; error bars represent standard deviations).
| Variables; Time Point | Mean ± SD | 95% CI | P-Value |
|---|---|---|---|
| GAIS (wrinkle severity) | 0.05 | ||
| Baseline | 10.8 ± 3.1 | 9.2, 12.4 | |
| One month post | 8.8 ± 2.9 | 7.3, 10.3 | |
| TTIS score | 0.001 | ||
| Baseline | 29.2 ± 4.5 | 27.0, 31.4 | |
| Immediately post | 37.6 ± 4.2 | 35.5, 39.7 | |
| One month post | 41.8 ± 3.9 | 39.8, 43.8 |
Abbreviations: GAIS, Global Aesthetic Improvement Scale; TTIS, Tear Trough Improvement Scale.
Participants' high-resolution photographs taken before and one month post-treatment demonstrated obvious refinement of the infraorbital area. Figure 5 depicts such a characteristic case. No significant adverse events, including prolonged edema, were reported, suggesting the SCEDIS-MSCS HA filler's suitability for the sensitive tear trough region.
5. Discussion
The innovative SCEDIS-MSCS HA filler demonstrated statistically significant improvement in tear trough deformity, as evidenced by GAIS scores, high-resolution photography, and the TTIS Questionnaire (7, 8). These findings align with prior studies on HA fillers for tear trough correction, which highlight the importance of filler quality in minimizing complications like edema (9, 10). The sustained satisfaction at one month, as shown in Figure 4, likely reflects the filler’s smooth integration and stability, attributed to the SCEDIS-MSCS technology’s ultrafine HA Neo-Spheres (5). Unlike traditional HA fillers, which may cause short-term edema or irregularities due to polydensified structures, the monophasic, mono-densified design of this filler minimizes such side effects, as evidenced by the absence of significant adverse events in this study (4, 9). The gradual improvement in patient satisfaction (TTIS) suggests that the filler’s tissue integration enhances over time, potentially due to its ability to provide a stable scaffold for cellular adhesion and hydration (7). However, the lack of a control group introduces potential biases such as placebo effects or natural aging, which should be addressed in future studies.
5.1. Limitations
This pilot study has limitations, including a small sample size (n = 16), a focus on Caucasian females with Fitzpatrick type III-IV skin, and the absence of a control group, which limits conclusions about the SCEDIS-MSCS HA filler’s specific efficacy compared to other treatments. The one-month follow-up may not capture long-term outcomes, and while GAIS and photography provide robust objective measures, additional metrics (e.g., skin hydration, collagen density) could enhance evaluation. These limitations are typical of pilot studies assessing initial efficacy and feasibility.
5.2. Future Research
A multicenter RCT is planned, targeting 100 participants (50% male, diverse skin types) with a placebo control and active comparator (e.g., standard HA filler). Follow-up assessments at 6 and 12 months will evaluate durability, with additional endpoints like skin hydration and tissue integration via imaging. This will address current limitations and provide robust evidence for the SCEDIS-MSCS HA filler’s efficacy.
![Results of subjective satisfaction feeling [Tear Trough Improvement Scale (TTIS) Questionnaire]; bar charts showing mean satisfaction scores (scale 8 - 40) at baseline, immediately post-treatment (P = 0.018), and one-month post-treatment (P = 0.001; error bars represent standard deviations). Results of subjective satisfaction feeling [Tear Trough Improvement Scale (TTIS) Questionnaire]; bar charts showing mean satisfaction scores (scale 8 - 40) at baseline, immediately post-treatment (P = 0.018), and one-month post-treatment (P = 0.001; error bars represent standard deviations).](https://services.brieflands.com/cdn/serve/3170e/551e769a7c05b0955ff8b21406d5b844083022fd/jssc-12-2-163854-i004-preview.webp)
