1. Background
Androgenetic alopecia (AGA) is an androgen-dependent, genetically mediated, autosomal dominant disorder with polygenic inheritance (1). It is a type of non-scarring alopecia characterized by the conversion of terminal hair to vellus hair due to increased end-organ hyperreactivity to androgens (2). It affects both genders but is more common in men, affecting 50% (3) as compared to 30% in females (4). High androgen levels contribute to the development of atherosclerosis and thrombosis, leading to hypertension and hypercholesterolemia (5). Studies in the past have demonstrated an association between AGA and metabolic syndrome or its associated diseases, including cardiovascular diseases, hypertension, and insulin resistance (6). Due to cosmetic concerns, the progressive nature of the disease, prolonged medical treatment, and unsatisfactory treatment responses, AGA has become a source of psychological distress for patients.
Numerous studies have been conducted seeking an association between male AGA and hyperlipidemia, but these lack substantial evidence, consistency, and uniformity (6). Fewer studies have been conducted in females. However, the precise magnitude of the problem remains difficult to measure. Hence, this study was undertaken to assess the frequency of dyslipidemia in both males and females with AGA.
2. Objectives
The objective of this study is to determine the frequency of dyslipidemia in males and females with AGA, and to measure the levels of total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides (TGs), TC/HDL ratio, and LDL/HDL ratio in patients with AGA.
3. Methods
From January 2020 to June 2021, a cross-sectional study was conducted on 90 patients attending the Dermatology Outpatient Department (OPD) of KR Hospital, affiliated with Mysore Medical College and Research Institute, Mysuru.
1. Inclusion criteria:
- Male and female patients between 18 - 55 years of age with AGA.
- Patients willing to undergo required investigations and treatment.
2. Exclusion criteria:
- Patients on systemic steroids, testosterone, or lipid-lowering agents.
- Patients with HIV, other immunocompromised states, diabetes mellitus, or hypertension.
- Previously treated cases of AGA.
- Patients with familial dyslipidemia, chronic alcoholism, hypothyroidism, or long-term retinoid therapy.
A detailed history was taken regarding the duration of patterned hair loss, family history, prior treatments, drug intake other than those for patterned hair loss, occupation, personal history of diabetes, hypertension, smoking, and alcohol intake. General physical and cutaneous examinations were performed for all patients. Male patients were graded according to the Modified Norwood-Hamilton Scale (7) for AGA, and female patients were graded with Ludwig’s classification (8). All changes involving nails, scalp, and mucosa were documented.
A fasting venous blood sample was collected after 8 - 10 hours of overnight fasting. Serum lipid levels including TC, TG, HDL, LDL, TC/HDL ratio, and LDL/HDL ratio were measured and recorded on a predesigned proforma for analysis and interpretation. Serum TC was measured using the enzymatic end point cholesterol oxidase method. Serum total TGs were estimated using the enzymatic method (GPO: PAP method). High-density lipoprotein was measured using the direct method. Very-low-density lipoprotein (VLDL) was estimated using the formula: VLDL = TG/5. Low-density lipoprotein was estimated using the Friedewald formula: LDL = TC - (VLDL + HDL) or LDL = TC - HDL - TG/5.
Summary statistics were calculated as proportions for categorical/binary variables and as mean, median, standard deviation, and interquartile range (IQR) for continuous variables. Inferential statistics were performed using the chi-square test/Fisher exact test, independent t-test, and one-way ANOVA. All statistical methods were conducted using SPSS 21.0 version for Windows. P < 0.05 was considered statistically significant.
The chi-square test/Fisher exact test was used to compare two or more independent proportions. The Fisher exact test was used when the number of expected values in > 20% of cells was < 5. The independent t-test was used to compare means between independent or mutually exclusive groups. One-way ANOVA was used to compare differences in means between multiple independent groups. A post-hoc test within one-way ANOVA was used for intergroup comparisons.
4. Results
A total of 46,000 cases attended the OPD during the study period, of which AGA constituted 1200 cases, representing 2.60% of all skin conditions.
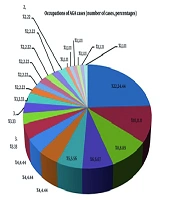
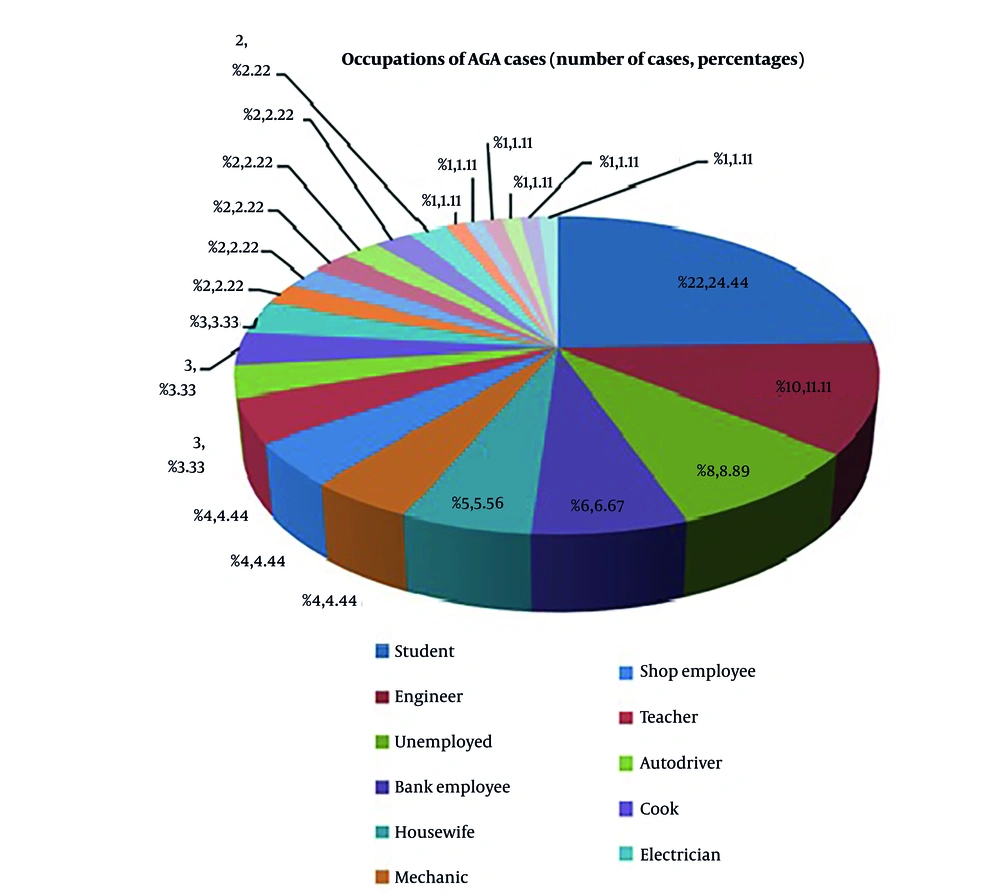
Out of 90 cases, 42.2% (38 cases) were under 25 years old. The remaining cases were in the 26 - 35 (35.6%), 36 - 45 (10%), and 46 - 55 (12.2%) age groups. The youngest patient was 18 years old, and the eldest was 55 years old. The mean age of cases was 30.4 years, and the male-to-female ratio was 3.09:1 (68 males and 22 females). Data are shown in Figure 1, which presents the correlation between occupation and AGA among study cases.
Family history was positive in 32 cases (35.6%), and alcohol consumption was seen in 16 cases (17.8%). Of the patients, 55 (61.1%) had a duration of hair loss of less than 1 year, 30 (33.3%) had 1 - 5 years, and 5 (5.6%) had more than 5 years.
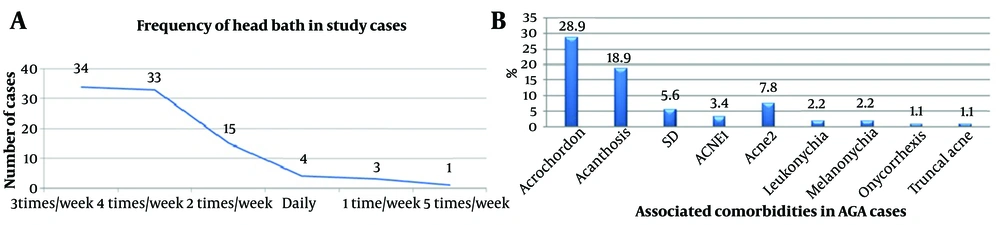
Of 68 male cases, 3 (4.411%) were AGA grade I, 30 (44.11%) were grade II, 14 (20.588%) were grade III, 9 (13.23%) were grade III vertex, 8 (11.76%) were grade IV, and 2 cases (2.941%) each were grade V and grade VII. No cases of grade VI were observed. Out of the 22 females, 11 (50%) each belonged to Ludwig’s grade I and II, and no cases were observed in grade III. The frequency of head bath among study cases and the comorbidities associated with AGA cases are depicted in Figure 2A and B, respectively.
Out of 90 cases, increased levels of TC were observed in 15 (16.7%), TGs in 38 (42.2%), LDL in 31 (34.4%), VLDL in 21 (23.3%), and the TC/HDL ratio in 34 (37.8%). Additionally, 57 cases (63.3%) had low HDL levels. Data are shown in Tables 1 and 2, which present the correlation of lipid levels with AGA grading in males and females, respectively.
| AGA Grade | TC (No., Mean ± SD) | TGA | HDL | LDL | TC/HDL | VLDL |
|---|---|---|---|---|---|---|
| I | 3, 124.0 ± 28.7 | 113.1 ± 39.2 | 35.6 ± 5.7 | 65.8 ± 25.4 | 3.5 ± 0.6 | 22.6 ± 7.8 |
| II | 30, 159.0 ± 30.2 | 134.7 ± 56.3 | 37.3 ± 6.7 | 92.8 ± 31.2 | 4.9 ± 2.9 | 27.1 ± 12.2 |
| III | 14, 173.1 ± 38.1 | 146.3 ± 72.8 | 34.9 ± 6.0 | 107.7 ± 34.1 | 5.1 ± 1.0 | 29.6 ± 13.4 |
| III vertex | 9, 163.1 ± 46.1 | 208.2 ± 147.1 | 49.5 ± 36.8 | 80.4 ± 25.8 | 4.2 ± 1.3 | 39.9 ± 30.4 |
| IV | 8, 178.8 ± 25.7 | 182.6 ± 40.8 | 34.4 ± 3.6 | 106.0 ± 27.3 | 5.5 ± 1.0 | 35.0 ± 12.8 |
| V | 2, 225.2 ± 25.0 | 147.5 ± 24.7 | 32.8 ± 4.5 | 110.6 ± 42.8 | 7.1 ± 1.6 | 77.9 ± 77.9 |
| VII | 2, 209.5 ± 18.0 | 173.2 ± 117.7 | 33.8 ± 5.6 | 149.2 ± 58.5 | 6.3 ± 1.6 | 26.5 ± 35.0 |
| P | 0.02 | 0.2 | 0.3 | 0.04 | 0.4 | 0.02 |
Abbreviations: AGA, androgenetic alopecia; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein.
a Values are expressed as mean ± SD.
| AGA Grade | TC | TGA | HDL | LDL | TC/HDL | VLDL |
|---|---|---|---|---|---|---|
| I | 11, 160.0 ± 31.4 | 123.0 ± 61.7 | 41.1 ± 12.3 | 92.5 ± 26.8 | 4.1 ± 1.2 | 26.2 ± 16.1 |
| II | 11, 181.9 ± 40.6 | 171.1 ± 82.9 | 41.6 ± 8.0 | 102.1 ± 37.5 | 7.7 ± 10.8 | 30.8 ± 18.7 |
| III | 0, 0 | 0 | 0 | 0 | 0 | 0 |
| P | 0.2 | 0.1 | 0.9 | 0.5 | 0.3 | 0.6 |
Abbreviations: AGA, androgenetic alopecia; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein.
a Values are expressed as mean ± SD.
Among 26 cases of acrochordons, 12 cases (46.2%) had increased TG levels (P = 0.6), 12 cases (46.2%) had increased LDL levels (P = 0.14), 7 cases (26.9%) had increased TC levels (P = 0.1), 7 cases (26.9%) had increased VLDL levels (P = 0.6), and 13 cases (50%) showed increased TC/HDL ratio (P = 0.1). Seventeen cases (65.4%) had decreased HDL levels (P = 0.8).
Among 17 cases of acanthosis nigricans, 12 cases (70.6%) had increased TG levels (P = 0.009), 11 cases (64.7%) had increased LDL levels (P = 0.004), 7 cases (41.2%) had increased TC levels (P = 0.003), 9 cases (52.9%) had increased VLDL levels (P = 0.001), and 11 cases (64.7%) showed increased TC/HDL ratio (P = 0.011). Twelve cases (70.6%) had decreased HDL levels (P = 0.05).
Among 5 cases of seborrheic dermatitis, 3 cases (60%) had increased TG levels (P = 0.4), 2 cases (40%) had increased LDL levels (P = 0.8), no cases had increased TC levels (P = 0.3), 2 cases (40%) had increased VLDL levels (P = 0.4), and 3 cases (60%) had increased TC/HDL ratio (P = 0.3). Four cases (80%) had decreased HDL levels (P = 0.4).
Among 3 cases of acne grade I, all 3 showed normal TG levels (P = 0.1), 1 case (33.3%) had increased LDL levels, and no cases showed changes in TC levels (P = 0.4) or VLDL levels (P = 0.3). Two cases (66.7%) showed increased TC/HDL ratio (P = 0.3), and 2 cases (66.7%) had decreased HDL levels (P = 0.9).
Among 7 cases of acne grade II, 5 cases (71.4%) had increased TG levels (P = 0.1), 2 cases (28.6%) had increased LDL levels (P = 0.7), 1 case (14.3%) had increased TC levels (P = 0.86), 3 cases (42.9%) had increased VLDL levels (P = 0.2), and 2 cases (28.6%) had increased TC/HDL ratio (P = 0.6). All 7 cases (100%) (P = 0.04) had decreased HDL levels.
5. Discussion
The prevalence of dyslipidemia in our study was 81.1%, which is consistent with other studies (1, 6, 9). However, it was higher than the 53.3% observed by Manoharan and Thomas (10), possibly due to differences in environmental, lifestyle, dietary, and genetic factors.
The mean age of onset of AGA in our study was 30.41 years, similar to studies by Qazi et al. (34.39 years) (6) and Lee et al. (28 years) (11). However, it was lower than Tehranchinia et al (12). (44.6 years) but higher than other Indian studies by Devi et al. (23 years) (13) and Sanke et al. (24 years) (14). These differences likely reflect varying age ranges in study populations and strong genetic factors.
In our study, the maximum number of cases belonged to the < 25 years age group (38 cases), followed by 26 - 35 years (32 cases), similar to Devi et al. (13), who also found a peak in an early age group (23 - 32 years; 23 cases). However, our study differed from Bilquees et al. (46 - 55 years; 49 cases) (1). Differences in age distribution in other studies are probably due to varying age ranges in the study populations. The early age of involvement in our study may be attributed to environmental factors, genetic predisposition, and lifestyle.
Among 68 males, the majority (41.2%) were < 25 years, followed by 26 - 35 years (35.3%), similar to Devi et al. (13) (62.9% between 23 - 27 years) but differing from Bilquees et al. (1), who found the highest incidence among males aged 46 - 55 years (32.6%) followed by 36 - 45 years (44 cases). Out of 22 female cases, the majority (45.5%) were < 25 years, unlike Tehranchinia et al. (12), who reported the highest number of female cases (33%) between 46 - 55 years. This difference in distribution among age groups may be due to genetic and environmental factors.
Incidence of AGA was higher in males (68 cases) than females (22 cases), which aligned with other studies (7, 15). Both sets of study observations outnumbered female cases, possibly because females may also have associated telogen effluvium, resulting in fewer being identified with AGA.
In our study, the majority of cases belonged to the student group (24.4%; 22 cases) followed by engineers (11.1%), the unemployed (8.9%), and bank employees (6.7%). However, Devi et al. (13) observed the highest incidence among the working population (55.5%) followed by students (44.4%). The higher proportion of student cases in our study may be due to day-to-day stress, altered dietary habits, and lifestyle factors. We observed a significant family history in 32 cases (35.6%), similar to the majority of studies (5, 11, 12), which highlights the influence of genetic factors among AGA cases.
Although alcohol consumption was seen in 16 cases (17.8%), the P-value was not significant, similar to Arias-Santiago et al. (9). However, a study by Sinclair (16) found a significant correlation. This could be due to the predominance of younger age groups in our study, who are less likely to have developed alcohol consumption habits compared to other countries. The frequency of head bath showed an insignificant P-value, possibly due to the small sample size; a larger sample may be necessary to clarify this finding.
The majority of cases had a duration of hair loss of less than 1 year, whereas Devi et al. (13), Banger et al. (5), and Arias-Santiago et al. (9) found most cases with 1 - 5 years or more than 5 years duration. The shorter mean duration in our study is likely due to the inclusion of more young patients with greater cosmetic concerns. In most studies, including our own, the majority of AGA cases were found in grade II, followed by grades III and IV. This differed from Banger et al. (5), who observed the most cases in grade III, followed by grade II, with an equal incidence in grades I and IV. However, in most Indian studies (6, 12), the majority of AGA cases fell within grade II, followed by grade III.
In our study, equal numbers of female cases (50% each) belonged to Ludwig’s grade I and II. Olsen (17) found more grade I cases (68.7%) followed by grade II (28.2%), while Arias-Santiago et al. (9) observed more grade II cases (58%). In both studies (9, 17), the severity of AGA among cases was either Ludwig’s grade I or II, which aligns with our findings.
We found an insignificant P-value (0.07) for TC levels among cases, similar to Manoharan and Thomas (0.759) (10) and Tehranchinia et al. (0.24) (12). However, significant P-values for TC were noted by other studies (5, 9). This discrepancy may be due to a higher proportion of younger patients and a smaller study population in our research.
In our study, TG levels were significantly increased in the 26 - 35 years (53.1%) and 46 - 55 years (63.6%) age groups, consistent with other studies (1, 5, 9). However, Tehranchinia et al. (12) observed insignificant P-values, differing from our findings.
The P-value for HDL levels was statistically significant (0.025), similar to studies by Bilquees et al. (P < 0.05) (1), Qazi et al. (0.0011) (6), and Arias-Santiago et al. (P < 0.0001) (9). However, other studies (5, 10, 14) found insignificant P-values for HDL levels. Deranged HDL may be an early indicator of dyslipidemia in younger populations.
While our study found no significant percentage of increased LDL levels for those under 45 years, there was a significant percentage increase in the 46 - 55 age group (63.6%), consistent with findings by Arias-Santiago et al. (9) and Qazi et al. (6), but insignificant in other studies (5, 10, 14).
A statistically significant increase in the TC/HDL ratio was observed in AGA cases in the 36 - 45 (55.6%) and 46 - 55 (63.6%) age groups. This finding, consistent with other studies (7, 10), suggests that the TC/HDL ratio becomes more significant with age, contrasting with the insignificant P-values found by Tehranchinia et al. (12).
Very-low-density lipoprotein levels showed no significant association among AGA cases (P = 0.24), similar to findings by Manoharan and Thomas et al. (P = 0.264) (10). Data are shown in Tables 3, and 4, which present the correlation of lipid levels and AGA grading in males and females, respectively.
| Studies | Mild-Moderate AGA P-Values b | Moderate-Severe AGA P-Values c | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TC | TGs | LDL | HDL | TC/HDL Ratio | VLDL | TC | TGs | LDL | HDL | TC/HDL Ratio | VLDL | |
| Present study | 0.002 | 0.2 | 0.04 | 0.3 | 0.4 | 0.02 | - | - | - | - | - | - |
| Qazi et al. (6) | 6.67 | 6.67 | 5 | 5 | - | - | 30, P = 0.0041 | 30, P = 0.0041 | 30, P = 0.0011 | 30, P = 0.0011 | - | - |
| Arias-Santiago et al. (9) | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | - | - | - | - | - | - | - |
| Gok et al. (18) | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | - | - | - | - | - | - | - |
Abbreviations: AGA, androgenetic alopecia; TC, total cholesterol; TGs, triglycerides; LDL, low-density lipoprotein; HDL, high-density lipoprotein; VLDL, very-low-density lipoprotein.
a Significant P-value: < 0.05.
b Grades I - IV.
c Grades V, VI, and VI.
Abbreviations: AGA, androgenetic alopecia; TC, total cholesterol; TGs, triglycerides; LDL, low-density lipoprotein; HDL, high-density lipoprotein; VLDL, very-low-density lipoprotein.
a Significant P-value: < 0.05.
b Grades I and II.
c Grade III.
As shown in Table 3, we found a statistically significant association between the severity of AGA and lipid levels in males for TC (P = 0.02), LDL levels (P = 0.04), and VLDL levels (P = 0.02), consistent with the findings of Qazi et al. (6). Other studies (9, 18), however, showed no significant correlation. No significant correlation was found in females (Table 4 P > 0.05), possibly due to the smaller sample size from simple random sampling, which is consistent with other studies (9, 18).
In our study, common comorbidities included acanthosis nigricans, acrochordons, seborrheic dermatitis, and acne grades I and II. Among comorbidities, acanthosis nigricans was significantly higher in patients with increased TC, TG, LDL, TC/HDL ratio, and VLDL levels, consistent with findings from other studies (19-21). Low HDL levels were statistically significant in patients with acne grade II, agreeing with results by EL-Akawi et al. (22) and Vergani et al. (23).
5.1. Conclusions
Our study demonstrated a statistically significant increase in TGs, LDL levels, TC/HDL ratio, and low HDL levels in the majority of cases. The variation in levels of TC, LDL, and VLDL was statistically significant among male cases with severe grades of AGA compared to mild to moderate grades. Cases of AGA with acne and acanthosis nigricans showed significant dyslipidemia in our study. The present study showed an increase in the prevalence of lipid abnormalities in AGA cases, which aligns with the majority of other studies reported from India and other countries. Hence, we recommend routine lipid profile screening for all patients presenting with AGA, particularly males, along with lifestyle modifications such as diet, exercise, avoidance of risk factors, and treatment of hyperlipidemia to minimize the risk of cardiovascular diseases such as atherosclerosis and its complications in these patients.
5.2. Limitations
Our study has several limitations, such as a small sample size — especially among female patients — and a single-center design, which may affect the generalizability of the results. We observed no significant correlation between alcohol consumption, frequency of head baths, and AGA; however, a larger sample size may be needed to determine the correlation of these factors with the severity of AGA. We encourage future large-scale and longitudinal studies to better clarify the causal relationship between AGA and dyslipidemia and to explore these associations in diverse populations.