1. Context
Chronic kidney disease (CKD) affects more than 850 million people globally and is associated with multiple dermatological complications, particularly xerosis and pruritus, which affect 40 - 90% of patients undergoing hemodialysis (HD) (1-4). These symptoms substantially impair quality of life, disturb sleep, increase psychological distress, and have been linked to higher mortality (5, 6).
The pathophysiology of uremic xerosis and chronic kidney disease-associated pruritus (CKD-aP) is multifactorial, involving impaired skin barrier function, reduced sebaceous and sweat gland activity, accumulation of uremic toxins and inflammatory mediators, opioid receptor dysregulation, mineral-bone disorder, and xerosis-induced stimulation of cutaneous nerve fibers (7, 8).
Management typically includes optimization of dialysis, correction of metabolic abnormalities, systemic agents such as antihistamines and gabapentinoids, and topical emollients (9-11). However, conventional moisturizers often provide limited relief, and systemic therapies may cause adverse effects in patients with CKD.
1.1. Virgin Coconut Oil: Properties and Potential Mechanisms
Virgin coconut oil (VCO), obtained through cold-pressing fresh coconut meat, contains approximately 90% saturated fatty acids — primarily medium-chain fatty acids such as lauric, capric, and caprylic acids — along with polyphenols, tocopherols, and phytosterols (12-14). These constituents support several mechanisms relevant to CKD dermatoses:
- Barrier repair: The VCO lipids resemble natural skin lipids, enhancing stratum corneum hydration and reducing transepidermal water loss (15, 16).
- Antimicrobial action: Lauric acid and monolaurin demonstrate broad-spectrum antimicrobial activity, with potential relevance in reducing Staphylococcus aureus colonization common in CKD patients (17, 18).
- Anti-inflammatory effects: Polyphenolics and antioxidants may modulate inflammatory mediators implicated in pruritus (19, 20).
- Practical advantages: The VCO is inexpensive, culturally acceptable, shelf-stable, and widely available, making it attractive for use in resource-limited settings (21).
1.2. Knowledge Gap and Need for Systematic Review
Despite increasing interest in VCO for dermatological care, evidence specific to uremic xerosis and CKD-aP remains fragmented, heterogeneous, and methodologically variable. Existing reviews have focused on general dermatology or non-CKD populations, leaving uncertainty regarding VCO’s effectiveness, safety, and optimal use in patients with CKD (22, 23). A focused systematic synthesis of available evidence is therefore warranted.
2. Objectives
This systematic review aimed to:
- Evaluate the efficacy of topical VCO in improving uremic xerosis and CKD-aP.
- Assess its safety profile and adverse events.
- Compare VCO with standard topical treatments.
- Identify optimal formulations and application protocols.
- Highlight evidence gaps to inform future research.
3. Methods
This systematic review followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 guidelines (24). The review protocol was not prospectively registered.
3.1. Eligibility Criteria
1. Inclusion criteria:
- Population: Adults (≥ 18 years) with CKD stages 3 - 5 or end-stage renal disease on HD or peritoneal dialysis presenting with uremic xerosis or CKD-aP.
- Intervention: Topical VCO or VCO-based formulations.
- Comparators: Mineral oil, standard lotions, other natural oils, usual care, or no comparator (single-arm studies).
- Outcomes: Xerosis [e.g., overall dry skin score (ODSS) and skin hydration] and pruritus severity [e.g., Visual Analog Scale (VAS), Numerical Rating Scale (NRS), and 5-D Itch]. Secondary outcomes included quality of life, adherence, satisfaction, and adverse events.
- Study designs: Randomized controlled trials (RCTs), quasi-experimental studies, cohort studies, case-control studies, pre-post studies, and case series (≥ 5 participants).
- Language: No restrictions.
2. Exclusion criteria: Systemic or oral VCO use; pediatric populations; non-CKD dermatological conditions; studies unrelated to xerosis or pruritus; abstracts and commentaries without data; and duplicate publications.
3.2. Information Sources and Search Strategy
Searches were conducted in PubMed/MEDLINE, Scopus, Embase, Google Scholar (first 200 results), and SciSpace from inception to November 8, 2025. Medical Subject Headings (MeSH) and free-text terms related to VCO, CKD, xerosis, pruritus, and topical therapy were combined.
Example PubMed search: [("Virgin Coconut Oil" OR “VCO” OR “Cocos nucifera” OR “coconut oil”) AND (“chronic kidney disease” OR “uremia” OR “renal insufficiency” OR “hemodialysis” OR “dialysis” OR “CKD” OR “ESRD”) AND (“xerosis” OR “pruritus” OR “dry skin” OR “itching”)]. Additional sources included reference list screening, citation tracking, and expert consultation.
3.3. Selection Process
Records were imported into a reference manager and deduplicated. Two independent reviewers screened titles and abstracts, followed by full-text assessment using predefined criteria. Disagreements were resolved by discussion or consultation with a third reviewer. Reasons for exclusion were documented. No automation tools were used.
3.4. Data Collection Process
Two reviewers independently extracted data using a standardized form. Discrepancies were resolved through consensus. Authors were contacted for missing data, but no responses were received.
3.5. Data Items
Extracted information included:
- Study characteristics: Author, year, country, design, sample size, demographics, CKD stage, dialysis modality, baseline xerosis or pruritus severity.
- Intervention details: The VCO formulation, application frequency, duration, anatomical site, and co-interventions.
- Comparators: Type and application protocol.
- Outcomes: The ODSS, skin hydration, VAS/NRS/5-D Itch, quality of life tools, sleep quality, adherence, and adverse events.
- Results: Baseline and post-intervention values, between-group differences, P-values, and effect sizes.
- Methodological details: Randomization, blinding, missing data handling, statistical analysis, and funding.
3.6. Risk of Bias Assessment
Two reviewers independently assessed study quality:
1. The RCTs: Cochrane Risk of Bias 2.0 tool (25), evaluating randomization, deviations from intended interventions, missing outcome data, outcome measurement, and selective reporting.
2. Quasi-experimental and observational studies: Joanna Briggs Institute (JBI) checklist (26), assessing group comparability, confounding control, presence of comparison groups, outcome measurement, follow-up completeness, and statistical methods.
Each domain was rated as low risk, some concerns, or high risk. Overall quality was categorized accordingly.
3.7. Effect Measures
Continuous outcomes were summarized using mean differences (MDs), standardized mean differences (SMDs), or percent change. For dichotomous outcomes, relative risks (RRs) or odds ratios (ORs) with 95% confidence intervals were used when available.
3.8. Synthesis Methods
Substantial heterogeneity in study designs, intervention protocols, comparators, outcome scales, and follow-up periods precluded meta-analysis. A structured narrative synthesis was performed, organized by:
- Study design (RCTs, controlled quasi-experimental studies, and uncontrolled pre-post studies).
- Outcome type (xerosis versus pruritus).
- Comparator category (mineral oil, standard lotions, other oils, and no treatment).
The synthesis included descriptive summaries, tables of study characteristics, and qualitative assessment of result consistency and potential sources of heterogeneity (CKD stage, dialysis modality, baseline severity, and intervention duration).
3.9. Reporting Bias Assessment
Given the small number of studies (< 10 per comparison), formal publication bias assessment was limited. Selective reporting was evaluated by comparing prespecified and reported outcomes and noting discrepancies between protocols (when available) and publications.
3.10. Certainty Assessment
Certainty of evidence was evaluated using a narrative Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (27), considering risk of bias, inconsistency, indirectness, imprecision, and publication bias. Evidence was rated as high, moderate, low, or very low (Table 1).
| Outcome | Study Design | Limitations | Certainty |
|---|---|---|---|
| Xerosis improvement (VCO vs. mineral oil) | 1 RCT | Imprecision | Moderate |
| Xerosis (VCO vs. standard lotion) | 1 quasi-exp | High bias | Low |
| Pruritus (VCO vs. usual care) | Quasi-exp+pre-post | Bias, imprecision | Low-moderate |
| Pruritus (VCO vs. other oils) | 2 quasi-exp | Bias | Low |
| Safety | All studies | Short follow-up | Low |
Abbreviations: VCO, virgin coconut oil; RCT, randomized controlled trial.
4. Results
4.1. Study Selection
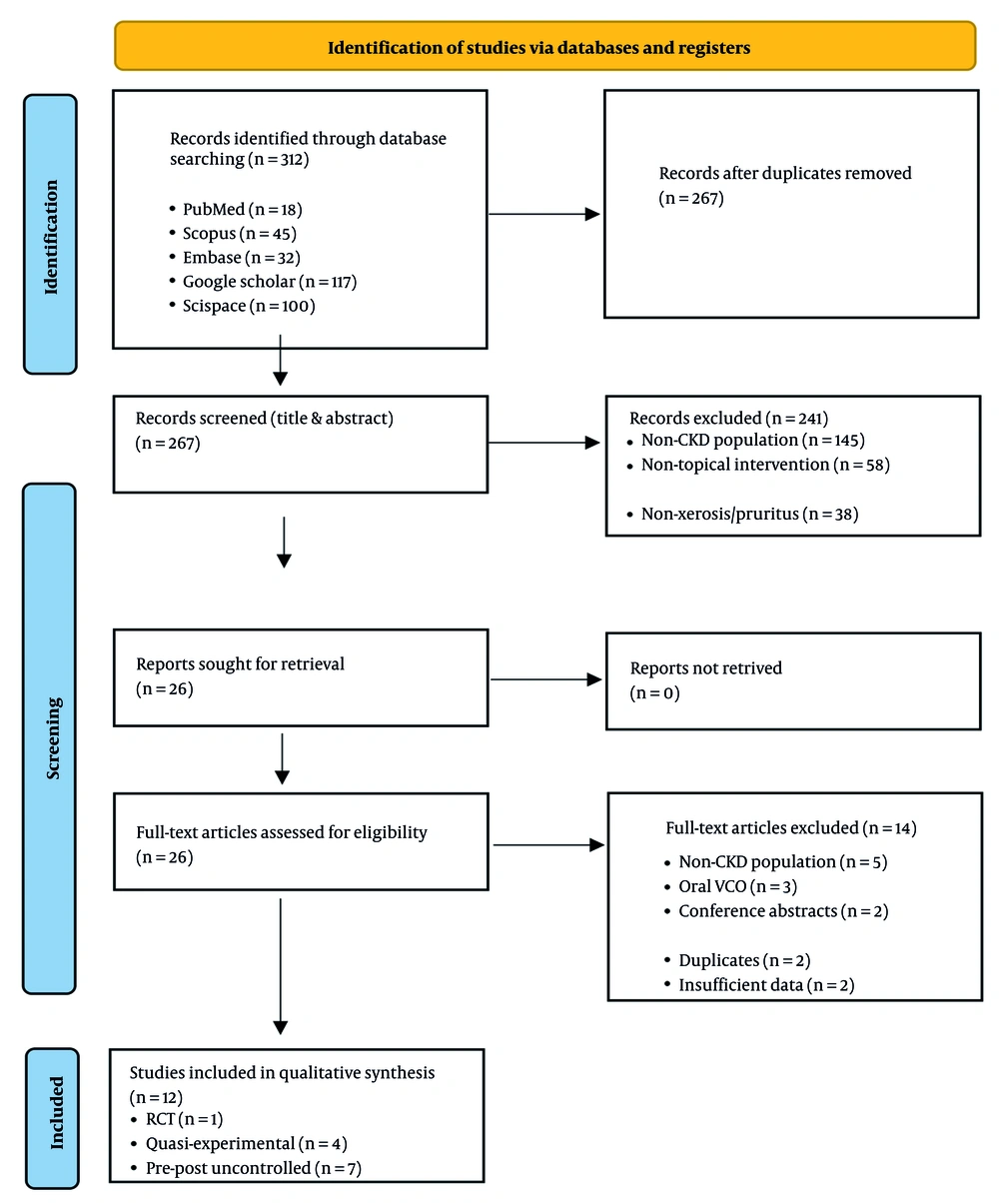
The database search yielded 312 records; 45 duplicates were removed, leaving 267 unique records for title and abstract screening. Of these, 241 were excluded for irrelevance. Twenty-six full-text articles were assessed, and 14 were excluded (non-CKD population n = 5; systemic VCO n = 3; conference abstracts n = 2; duplicates n = 2; insufficient data n = 2). Twelve studies met eligibility criteria and were included in the qualitative synthesis. The study selection process is presented in Figure 1.
Studies included in qualitative synthesis (n = 12):
- The RCT (n = 1).
- Quasi-experimental (n = 4).
- Pre-post/uncontrolled (n = 7).
4.2. Study Characteristics
The 12 included studies (2014 - 2023) were conducted mainly in Indonesia, the Philippines, and India.
- Study designs: One RCT, 4 quasi-experimental controlled studies, and 7 uncontrolled pre-post studies.
- Sample sizes: Approximately 350 participants in total (range 11 - 80).
- Population: Primarily adults with stage 5 CKD on HD with moderate to severe xerosis or pruritus.
- Interventions: All studies used pure VCO, typically applied 1 - 2 times daily for 1 - 4 weeks, primarily on extremities and trunk.
- Comparators: Mineral oil (n = 1), standard lotions (n = 2), natural oils such as Nigella sativa or olive oil (n = 3), or no comparator (n = 5; Table 2).
| Study (Author, y) | Country | Design | Sample Size | Population | CKD Stage/Dialysis | VCO Application Protocol | Comparator | Duration | Primary Outcomes | Key Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| De las Alas et al., 2014 (28) | Philippines | RCT | 60 (30/30) | HD patients with uremic xerosis | Stage 5/HD | Twice daily to extremities | Mineral oil | 1 wk | ODSS, patient assessment | VCO group: Significant ODSS improvement vs. mineral oil (P < 0.05) |
| Saodah et al., 2020 (29) | Indonesia | Quasi-experimental | 80 (40/40) | HD patients with xerosis | Stage 5/HD | VCO+lotion program, twice daily | Ordinary lotion | 2 wk | Skin moisture (objective measurement) | Both groups improved; ordinary lotion showed greater moisture normalization than VCO. |
| Melastuti and Setyaningrum, 2016 (30) | Indonesia | Quasi-experimental | 60 (30/30) | HD patients with pruritus | Stage 5/HD | VCO application protocol | Usual care | 2 wk | 5-D Itch Scale | Significant reduction in itch scores vs control (P = 0.000) |
| Ramadhani, [year] (31) | Indonesia | Quasi-experimental | 40 (20/20) | CKD patients with pruritus | Stage 4 - 5/HD | VCO topical application | Nigella sativa oil | 2 wk | Pruritus Scale | Both groups improved; no significant between-group difference (P = 0.754) |
| Verma and Gota, 2021 (32) | India | Comparative trial | ~40 | CKD patients with pruritus | Stage 4 - 5 | Coconut oil application | Liquid paraffin | 2 - 4 wk | Pruritus VAS | Both groups showed improvement; design details unclear. |
| Desnita and Sapardi, 2020 (33) | Indonesia | Pre-post | 11 | HD patients with xerosis | Stage 5/HD | Twice daily for 12 days | None | 12 d | ODSS | Mean ODSS improved from 3.06 to 1.39 (P = 0.001) |
| Daryaswanti et al., 2019 (34) | Indonesia | Pre-post | 52 | CRF patients | Stage 5 | VCO+cutaneous stimulation | None | 2 wk | Skin moisture, comfort, and sleep | Improved moisture, comfort, and sleep; reduced itch |
| Helnawati et al., 2023 (35) | Indonesia | Pre-post | 15 | HD patients with pruritus | Stage 5/HD | VCO massage protocol | None | 1 - 2 wk | NRS pruritus | Significant reduction in NRS scores post-massage |
| Muliani et al., 2021 (36) | Indonesia | Pre-post comparison | ~30 | HD patients with pruritus | Stage 5/HD | VCO vs olive oil (sequential) | Olive oil (within-subject) | 2 wk each | Pruritus grade scores | Both oils reduced pruritus; VCO showed slightly better outcomes |
| Abbasi et al., 2022 (37) | Iran | Pre-post | 41 | HD patients with pruritus | Stage 5/HD | Chia oil+coconut oil | None (combined intervention) | 4 wk | Pruritus VAS, lab parameters | Significant reduction in VAS scores; improved lab parameters |
| [Study 11]a | Indonesia | Pre-post | ~25 | HD patients | Stage 5/HD | VCO application | None | 2 wk | Xerosis/pruritus | Improvement in both outcomes |
| [Study 12] a | Indonesia | Pre-post | ~20 | HD patients | Stage 5/HD | VCO topical | None | 1 - 2 wk | Skin hydration | Increased moisture measurements |
Abbreviations: RCT, randomized controlled trial; HD, hemodialysis; VCO, virgin coconut oil; ODSS, overall dry skin score; NRS, Numerical Rating Scale; VAS, Visual Analog Scale; CRF, chronic renal failure; CKD, chronic kidney disease.
a These studies include systematic reviews sourced from gray literature, consisting of local unpublished Indonesian studies.
4.3. Risk of Bias in Studies
1. The RCT (n = 1): The single RCT had moderate risk of bias, mainly due to unclear allocation concealment and non-blinded outcome assessment.
2. Quasi-experimental studies (n = 4): Risk of bias was moderate to high, largely due to non-random allocation, potential confounders, and limited sample size justification.
3. Uncontrolled pre–post studies (n = 7): Risk of bias was high due to absence of control groups, short follow-up, potential placebo effects, and inconsistent outcome reporting.
Summary:
- One study (8%) = moderate risk.
- Four studies (33%) = moderate to high risk.
- Seven studies (58%) = high risk.
Overall confidence in the evidence is limited by methodological constraints.
4.4. Results of Individual Studies
4.4.1. Randomized Controlled Trial
De las Alas et al., 2014 (n = 60) compared VCO versus mineral oil for xerosis. The VCO improved ODSS by 53%, compared with 26% for mineral oil (P < 0.05). Eighty percent of VCO users reported “good/excellent” improvement versus 50% in the mineral oil group. No serious adverse events (conclusion: The VCO showed superior short-term efficacy) (28).
4.4.2. Quasi-experimental Controlled Studies
Saodah et al., 2020 (n = 80): Both VCO plus lotion and ordinary lotion improved moisture, but ordinary lotion performed better (P < 0.05) (29).
- Melastuti and Setyaningrum, 2016 (n = 60): The VCO reduced 5-D Itch scores by 50%, compared to 14% in usual care (P = 0.000) (30).
- Ramadhani (n = 40): The VCO and Nigella sativa oil produced similar pruritus reductions (~43 - 44%; P = not significant) (31).
- Verma and Gota, 2021 (n ≈ 40): Both coconut oil and liquid paraffin improved pruritus; methodology unclear (32).
- Conclusion: The VCO consistently improved xerosis and pruritus; comparative performance varied by comparator.
4.4.3. Uncontrolled Pre-Post Studies
Seven studies (n = 11 - 52) consistently showed: Forty to fifty-five percent reductions in xerosis or pruritus (ODSS, VAS, and NRS); improved skin hydration and comfort; no significant adverse events; and combined interventions (e.g., VCO plus cutaneous stimulation) limit attribution of effects in some studies.
4.5. Synthesis of Results
4.5.1. Efficacy for Uremic Xerosis
The RCT supports VCO superiority over mineral oil for ODSS improvement (moderate-certainty). Standard lotion outperformed VCO in one quasi-experimental study. Multiple pre-post studies showed marked xerosis improvements (~50%); overall: The VCO is effective for short-term xerosis relief; evidence versus modern lotions is mixed; certainty: Moderate for VCO versus mineral oil; low for other comparisons.
4.5.2. Efficacy for Chronic Kidney Disease-Associated Pruritus
The VCO reduced pruritus by 40 - 50% across controlled and uncontrolled studies. More effective than usual care, similar to Nigella sativa and olive oil. No studies compared VCO with pharmacological antipruritic agents; overall: The VCO shows meaningful short-term antipruritic effect; certainty: Low to moderate due to high quasi-experimental bias.
4.5.3. Safety and Adverse Events
Across all 12 studies:
- No serious adverse events.
- Mild oiliness in 10 - 15%.
- Occasional mild irritation (< 5%).
- No allergic reactions.
Limitations include short follow-up and inconsistent safety reporting; overall: The VCO appears safe for short-term use; long-term safety remains unclear; certainty: Low due to limited monitoring.
4.5.4. Optimal Application Protocols
Evidence supports:
- Pure VCO.
- Twice-daily application.
- Duration: 1 - 2 weeks.
- Sites: Extremities and trunk.
- No studies assessed formulation comparisons, dose standardization, or maintenance protocols.
4.5.5. Heterogeneity and Subgroup Considerations
Substantial heterogeneity existed in study designs, comparators, outcome measures, baseline severity, and climate. Most studies were conducted in tropical climates among stage 5 CKD patients on HD, limiting generalizability.
4.6. Reporting Biases
Formal publication bias assessment was not feasible. All published studies reported positive or neutral findings, raising the possibility of unpublished negative results. Selective outcome reporting was minimal, but adverse event reporting was inconsistent (overall: Evidence supporting VCO for CKD-related xerosis and pruritus is of low to moderate certainty, limited by small samples, short interventions, and risk of bias).
5. Discussion
5.1. Summary of Main Findings
This review included 12 studies (1 RCT, 4 quasi-experimental, 7 pre-post; ~350 participants) evaluating topical VCO for uremic xerosis and CKD-aP. Overall, the evidence suggests short-term benefits with a favorable safety profile, though methodological limitations restrict certainty.
1. Xerosis: The only RCT reported greater improvement with VCO than mineral oil (53% vs. 26%). Other studies consistently showed improvement, although one quasi-experimental study found standard lotion more effective than VCO for hydration.
2. Pruritus: The VCO produced meaningful reductions in pruritus (40 - 55%) across controlled and uncontrolled studies. Effects were comparable to Nigella sativa or olive oil but superior to usual care.
3. Safety: No serious adverse events were reported. Minor oiliness (10 - 15%) was the most common concern.
4. Evidence quality: Overall certainty was low to moderate due to small sample sizes, high risk of bias, short treatment durations (1 - 4 weeks), and heterogeneous outcome measures.
5. Knowledge gaps: Notable gaps include the absence of large RCTs, lack of long-term follow-up, insufficient comparisons with modern emollients, and limited generalizability beyond Asian HD populations.
5.2. Mechanisms of Action
1. Barrier restoration: The VCO’s medium-chain fatty acids (especially lauric acid) integrate into the stratum corneum, reducing transepidermal water loss and improving hydration. This mechanism aligns with xerosis pathophysiology in CKD, where sebaceous activity and lipid composition are impaired.
2. Anti-inflammatory effects: Although not measured in included studies, VCO contains polyphenols and tocopherols shown elsewhere to reduce oxidative stress and inflammatory mediators (e.g., IL-6, TNF-α). These mechanisms plausibly target neuroinflammatory pathways implicated in CKD-aP.
3. Antimicrobial properties: Lauric acid and monolaurin exhibit activity against S. aureus, a common colonizer in CKD that may worsen inflammation and barrier dysfunction. While untested in CKD studies, this may partly contribute to symptom improvement.
4. Sensory and psychological effects: Regular oil application and massage can disrupt the itch-scratch cycle and enhance comfort through gate-control mechanisms and increased self-efficacy.
5.3. Comparison with Existing Literature
Evidence from general dermatology supports VCO’s benefits for xerosis and atopic dermatitis. The present findings extend these effects to patients with CKD, who have distinct skin barrier deficiencies.
The CKD-related pruritus management typically emphasizes dialysis optimization, emollients, phototherapy, antihistamines, gabapentinoids, and emerging agents (e.g., difelikefalin). Within this therapeutic landscape, VCO may serve as a low-cost, accessible topical option. Comparative data from the natural oils literature show broadly similar emollient effects across oils, though VCO’s high lauric acid content may offer added antimicrobial benefits.
5.4. Clinical Implications and Practice Recommendations
1. Appropriate clinical use: The VCO may be considered as a first-line topical therapy for mild to moderate xerosis and pruritus, especially where standard emollients are unavailable or costly. Additionally, VCO may be considered as an adjunct to systemic treatments for moderate to severe pruritus.
2. Suggested protocol (based on current evidence):
- Formulation: Pure, cold-pressed VCO.
- Frequency: Twice daily.
- Sites: Affected areas (typically extremities, trunk).
- Duration: Initial trial of 1 - 2 weeks; continue if improved.
3. Patient selection and monitoring: Suitable for adults with CKD stages 3 - 5, including patients on dialysis; reassess symptoms at 1 - 2 weeks; monitor for irritation or folliculitis; discontinue if no improvement by 2 - 4 weeks.
4. Contraindications: Known coconut allergy; active skin infections or open wounds; folliculitis-prone individuals.
5. Patient education: Patients should understand that VCO is supportive — not curative; moderate improvement is typical; continued CKD and dialysis optimization remains essential.
6. Cost-effectiveness considerations: The VCO is inexpensive, widely available, and culturally acceptable in many regions, making it potentially cost-effective for low-resource settings. Formal economic evaluations are needed.
5.5. Strengths and Limitations
Strengths of this review include comprehensive search including regional studies, PRISMA-compliant methodology, dual screening and data extraction, and focus on clinically relevant outcomes for CKD patients. Additionally, limitations of this review include potential language and publication bias, limited access to grey literature, heterogeneity prevented meta-analysis, and some review steps conducted by a single reviewer. Moreover, limitations of the evidence base comprise high proportion of quasi-experimental and pre-post designs, small sample sizes and short intervention periods, variation in measurement tools, predominantly Southeast Asian settings limit generalizability, inconsistent reporting of safety outcomes, and few comparisons with contemporary standard moisturizers.
5.6. Implications for Research
Large, high-quality RCTs are urgently required. Key research priorities include:
1. Rigorous RCTs: Multi-centre, adequately powered trials (≥ 100 per arm), comparisons with ceramide- or urea-based moisturizers, follow-up ≥ 12 weeks with validated Xerosis and Pruritus scales, and robust safety monitoring.
2. Mechanistic studies: Transepidermal water loss, hydration, and skin lipid analysis; inflammatory markers and microbiome assessments; and neural and sensory pathway evaluation.
3. Formulation and protocol optimization: The VCO versus VCO-based lotions or emulsions, dose-response and frequency studies, and whole-body versus targeted application.
4. Comparative effectiveness research: Head-to-head trials with natural oils and standard emollients, and pragmatic studies in real-world CKD care.
5. Generalizability and subgroup studies: Pre-dialysis CKD, peritoneal dialysis, diverse climates, and ethnic groups.
6. Economic and long-term safety assessments: Cost-effectiveness analyses and longer follow-up (≥ 6 months), and large cohorts to detect rare adverse reactions.
5.7. Conclusions
This review indicates that topical VCO offers short-term benefits for xerosis and CKD-aP, with improvements of 40 - 55% and a favorable safety profile. One RCT demonstrated superiority over mineral oil, though evidence remains insufficient to establish equivalence with modern emollients.
The overall certainty of evidence is low to moderate, primarily due to small sample sizes, risk of bias, heterogeneous outcomes, and short follow-up durations. The VCO can be cautiously recommended as a low-cost, accessible topical option — particularly in resource-limited communities — and as part of a broader pruritus management strategy. Large, well-designed RCTs with standardized outcomes and long-term assessment are essential to define VCO’s definitive role in CKD dermatological care.