1. Context
Janus kinases (JAKs) are classified into four isoforms: JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2). These are intracellular enzymes associated with the intracellular domains of various cytokine receptors, initiating inflammatory responses upon antigenic stimulation (1). Baricitinib (Olumiant™) is a reversible, competitive inhibitor of the JAK1 and JAK2 family, functioning as an anti-inflammatory agent by blocking signal transduction of specific JAKs (2). The European Union approved baricitinib in February 2017 for rheumatoid arthritis, followed by United States Food and Drug Administration (FDA) approval in 2018 for the same indication. Among dermatological indications, it received FDA approval for moderate to severe alopecia areata (AA) in June 2022. This review highlights the therapeutic indications of baricitinib across cutaneous diseases, incorporating clinical trial data, real-world studies, and ongoing investigations assessing baricitinib’s efficacy, dose optimization, maintenance strategies, and long-term safety in dermatologic conditions.
2. Pharmacology of Baricitinib
Baricitinib (Olumiant™) is a small molecule with a molecular weight of 371.42 g/mol and the chemical formula C16H17N7O2S (3). Baricitinib is well absorbed orally, with approximately 79% bioavailability and peak plasma concentration achieved within one hour, unaffected by food intake. It exhibits 50% plasma protein binding, independent of concentration, and has an average volume of distribution of 1.1 L/kg, indicating moderate tissue penetration. The elimination half-life is 6 - 9 hours, which is prolonged in cases of severe renal impairment. Approximately 75% of clearance occurs through renal excretion, about 20% via feces, and the remainder through hepatic metabolism, primarily by the enzyme CYP3A4 (4).
3. Mechanism of Action
Baricitinib is a selective, reversible inhibitor of JAK1 and JAK2, with moderate to significant activity against TYK2, and minimal effect on the JAK3 receptor. The JAK-signal transducer and activator of transcription (STAT) pathway regulates intracellular signal transduction from extracellular cytokine stimulation to the cell nucleus (5). In response to antigens, cytokines bind to their receptors on the cell membrane, resulting in conformational changes that activate the associated JAK complexes. This activation leads to autophosphorylation and increased JAK activity, as well as phosphorylation of the intracellular domain of their receptor (6). This process creates a docking site for members of the STAT family, followed by phosphorylation of STAT members by JAKs. The phosphorylated STATs translocate to the nucleus, where they bind to specific DNA sequences and induce gene transcription, leading to inflammation. Thus, baricitinib exerts its anti-inflammatory action by blocking these signaling pathways (7).
4. Indications of Baricitinib
4.1. Alopecia Areata
The AA is an autoimmune disorder characterized by non-scarring hair loss, primarily driven by the breakdown of hair follicle immune privilege and subsequent CD8+ T-cell-mediated inflammation. Interferon-gamma (IFN-γ), mainly produced by CD8+ T-cells, activates JAK1/2 to enhance interleukin-15 (IL-15) production. The IL-15 binds to IL-15α receptors on hair follicle epithelium, stimulating CD8+ T-cells via JAK1/3 to release additional IFN-γ. This self-perpetuating cycle recruits more lymphocytes and drives follicular damage, ultimately causing hair loss. Baricitinib interrupts this pathway, preventing IFN-γ–driven inflammation (8). Currently, it is FDA approved for the treatment of moderate to severe AA at a dose of 2 mg to 4 mg per day (Box 1).
| FDA approved |
| Dermatological |
| Moderate to severe AA (June 2022) |
| Non-dermatological |
| Rheumatoid arthritis (June 2018) |
| COVID-19 infection (May 2022) |
| Off-label |
| AD (EMA approved for moderate to severe AD in adults > 18 years, in September 2020) |
| Psoriasis |
| Vitiligo |
| Subacute cutaneous lupus erythematosus |
| Cutaneous dermatomyositis |
| Diffuse cutaneous systemic sclerosis |
| Chronic pruritus of unknown origin |
| Systemic lupus erythematosus |
| Diabetic nephropathy |
| Refractory Juvenile dermatomyositis |
Abbreviations: AA, alopecia areata; AD, atopic dermatitis; EMA, European Medicines Agency; FDA, United States Food and Drug Administration.
Key findings from Recent Baricitinib Studies in AA:
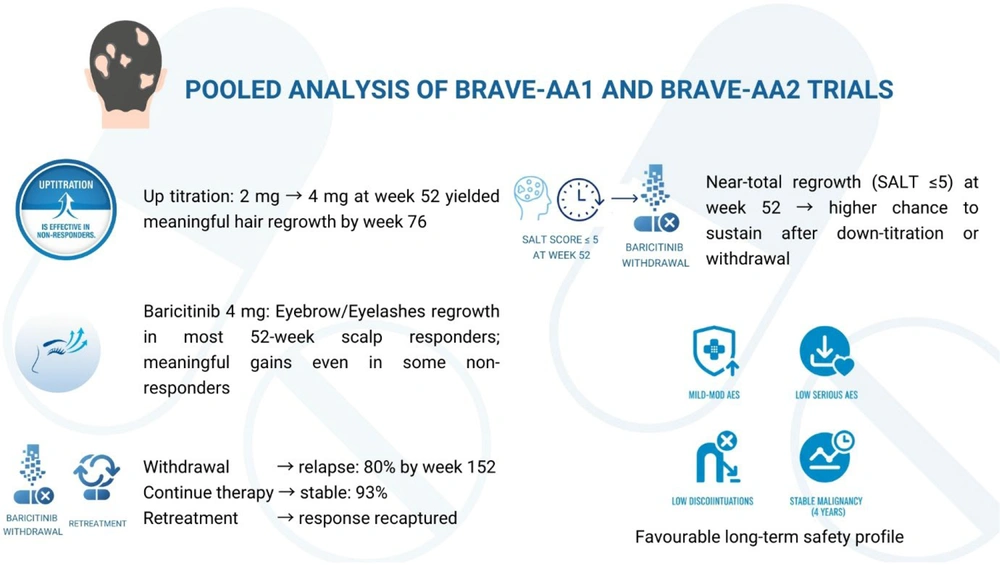
4.1.1. Pooled Analysis of BRAVE-AA1 and BRAVE-AA2 Trials
- Up-titration is effective in non-responders: Increasing the baricitinib dose from 2 mg to 4 mg in non-responders at week 52 led to meaningful benefits by week 76, with notable eyebrow and eyelash regrowth (9).
- Eyebrow/eyelash (EB/EL) regrowth: The EB/EL involvement strongly paralleled scalp alopecia severity. Baricitinib, particularly 4 mg, produced significant EB/EL regrowth, with most scalp responders by week 52 also demonstrating EB/EL recovery, supporting holistic treatment outcomes (10).
- Withdrawal and retreatment: In the BRAVE-AA1 withdrawal sub-study, up to 80% of patients who discontinued baricitinib lost clinical benefit by week 152, compared with only 7% among those who continued therapy. Initiating retreatment ensured clinical responses, underscoring AA’s relapsing course and the necessity of continuous treatment (11).
- Severity of Alopecia tool (SALT) ≤ 5 at 52 weeks can guide dose reduction strategy: In the BRAVE-AA2 down-titration sub-study, 88.6% of patients who continued 4 mg maintained responses at week 152 versus 58.5% on 2 mg. Patients with near-total regrowth (SALT ≤ 5) at week 52 were more likely to sustain benefit after dose reduction (12).
- Favorable long-term safety profile: Pooled long-term safety analysis of 1,303 patients (2,789.7 patient-years, median 825 days, maximum 1,460 days) showed most adverse events were mild to moderate, with a low incidence of serious events, discontinuations, or malignancies. No new safety concerns emerged through four years of exposure, confirming the favorable long-term safety profile of baricitinib (Figure 1) (13).
4.1.2. Benefits in Short- and Long-Standing Disease
Shorter-duration disease cohorts show greater baricitinib responsiveness (58% SALT ≤ 20 at 12 months; less than four-year episodes predict superior outcomes) (14). However, long-episode cohorts (greater than eight years) still achieve significant regrowth with favorable safety, expanding therapeutic evidence for refractory disease (15).
4.1.3. Therapeutic Burden as a Practical Stratification Tool
Lower therapeutic burden (fewer prior systemic treatments) predicted greater 12 month SALT improvement, independent of disease duration, severity, or sex (16). Considering baricitinib as a first-line treatment may improve outcomes.
4.1.4. Intra-class Switching to Address Inadequate Janus Kinases-Signal Transducer and Activator of Transcription Response
Baricitinib is effective and well-tolerated in pediatric and adult AA after tofacitinib inadequacy, including eyebrow and eyelash regrowth, supporting intra-class switching and rescue (17). Conversely, another retrospective cohort showed remission, maintenance, and improvement after switching mainly in partial/relapse cases, with minimal benefit for primary nonresponders, indicating prior response as a key predictor of switching success (18).
4.1.5. Safety in Pediatric Severe Alopecia Areata
In 33 children (ages 2 - 18) with severe AA (SALT ≥ 50), baricitinib given for an average of 6.5 months led to ≥ 50% SALT reduction in 45.5% of patients. Both 2 mg and 4 mg doses were effective, with only mild adverse events (elevated liver enzymes, acne, upper respiratory infection) and no serious events (19).
4.2. Atopic Dermatitis
Atopic dermatitis (AD) is a common chronic inflammatory skin disease with a relapsing-remitting course. Its pathogenesis is multifactorial, involving T helper 2 (Th2) cytokines [interleukin-4 (IL 4), IL-13, IL-31, IL-5], Th22 cytokines (IL-22), and Th1-related mediators such as thymic stromal lymphopoietin (TSLP). IL 5 binding to its receptor activates JAK1/JAK2, inducing STAT1, STAT2, and STAT5 signalling, while TSLP binding to IL-7 and TSLP receptors similarly activates JAK1/JAK2, driving STAT5 (20). By selectively inhibiting JAK1 and JAK2, baricitinib blocks downstream signalling cascades, thereby suppressing pathogenic immune activation and reducing inflammation. Baricitinib, either as monotherapy or combined with topical corticosteroids, provides significant improvement in symptoms and quality of life for patients with moderate-to-severe AD, demonstrating consistent efficacy across all evaluated dosages (21). Furthermore, baricitinib-loaded nanoemulgels show promise for AD by enhancing skin retention, inhibiting inflammation, and reducing severity, offering a potential topical alternative (22). Key findings from recent baricitinib studies in AD:
4.2.1. Rapid Eczema Area and Severity Index Subscore Gains
Baricitinib 4 mg rapidly improved all four EASI subscores (excoriation, edema/papulation, erythema, and lichenification) in moderate-to-severe AD, with significant effects from week 1, plateauing by week 4, and sustained through week 16. The most pronounced effect was on excoriation, indicating swift inflammation control and early itch-scratch cycle disruption (23).
4.2.2. Baricitinib Dosing Flexibility in Atopic Dermatitis Patients
After 52-week response, continuous dosing outperformed down-titration and drug withdrawal for maintaining achieved response. Loss of response was highest with withdrawal, yet most patients rapidly recaptured control upon reinitiation, supporting maintenance therapy with conditional, reversible step-down strategies (24).
4.2.3. Sustained Improvement in Quality of Life
In adults with moderate-to-severe AD entering BREEZE-AD3, continuous baricitinib 4 mg sustained improvements across clinician- and patient-reported outcomes through 104 weeks; dose down-titration to 2 mg preserved most gains, supporting flexible long-term maintenance with durable control of skin inflammation, itch, sleep disturbance, and quality of life (25).
4.2.4. Cost-Conscious Low-Dose Baricitinib
Low-dose baricitinib (2 mg daily) offers a cost-conscious entry point for systemic therapy in JAK inhibitor-naïve patients with moderate-to-severe AD. It produced rapid pruritus relief within 1 - 2 weeks and early EASI gains, with good tolerability and pragmatic acceptability (26).
4.2.5. Long-term Efficacy and Safety in Pediatric Atopic Dermatitis
In the BREEZE-AD-PEDS extension trial of 467 pediatric patients with moderate-to-severe AD, baricitinib showed sustained efficacy up to 3.6 years, with the 4 mg equivalent achieving the highest response rates. Most adverse events were mild, with no new safety concerns reported (27).
4.3. Psoriasis
Psoriasis, a chronic inflammatory skin disorder, involves interleukin-23/Th17 pathway activation via JAK2 and TYK2, making JAK inhibitors promising targeted therapies (28). Bhaskarmurthy and Evan Prince postulated that baricitinib reduces the phosphorylation of STAT3 and STAT1 levels, further inhibiting the expression of inflammatory cytokines (29). In a randomized, double-blind, phase 2b study, 8 - 10 mg doses produced significant efficacy, with Psoriasis Area and Severity Index (PASI)-75 responses in moderate-to-severe psoriasis at 12 weeks (30). However, baricitinib has lagged behind in the therapeutic race for psoriasis and psoriatic arthritis. Other JAK-STAT inhibitors such as tofacitinib, upadacitinib, and deucravacitinib have demonstrated efficacy comparable to established treatments, with upadacitinib and deucravacitinib already receiving FDA approval (31).
4.4. Vitiligo
Vitiligo is an acquired, multifactorial, chronic depigmenting disorder of the skin caused by melanocyte loss. The main pathogenic factor implicated is IFN-γ-related chemokines via the JAK-STAT pathway, especially CXCR3+ and CXCL-10. CXCR3+CD8+ T-cells on activation stimulate melanocyte destruction and apoptosis. Therefore, JAK inhibitors block the action of IFN-γ and CXCL-10 and represent a potential therapy for vitiligo (32). In vitro, baricitinib (25 μM) enhanced tyrosinase activity, stimulated melanin synthesis, and upregulated TYR and TRP 1 expression in ultraviolet B-damaged melanocytes, suggesting a role in restoring melanocyte function and mitigating pigment loss under oxidative stress (33). Across two complementary studies, combining oral baricitinib with narrow band-ultraviolet B therapy (NB-UVB) yielded clinically meaningful repigmentation and acceptable safety in progressive, nonsegmental vitiligo. In an open-label study, patients were assigned combination therapy with baricitinib 2 mg daily and NB-UVB three times a week or NB-UVB alone three times a week. Of the 33 patients, 70.6% in the combination group and 12.5% in the control group achieved a total Vitiligo Area Scoring Index (T-VASI50) response at week 16 (relative risk = 5.6; 95% confidence interval = 1.5 - 21.4; P = 0.001) (34).
4.5. Lupus Erythematosus
Baricitinib’s phase 3 systemic lupus erythematosus (SLE) program yielded mixed outcomes; a pooled analysis of SLE-BRAVE-I/II demonstrated no overall Systemic Lupus Erythematosus Responder Index (SRI-4) benefit at week 52 versus placebo. Nonetheless, efficacy signals emerged in prespecified subgroups of patients on baseline prednisone ≥ 10 mg/day and those with highly active disease, supporting targeted evaluation in enriched populations (35). Beyond systemic effects, anecdotal evidence supports baricitinib's utility for cutaneous SLE manifestations. One case reported complete resolution of treatment-refractory papulosquamous rash within four weeks of baricitinib 4 mg/day, enabling glucocorticoid discontinuation after failure of multiple immunosuppressants, including cyclosporin, methotrexate, and thalidomide (36).
4.6. Cutaneous Dermatomyositis
Baricitinib has emerged as a promising therapeutic option for refractory dermatomyositis (37). Clinical studies and anecdotal reports demonstrate its efficacy in ameliorating cutaneous manifestations, supported by a prospective open-label trial demonstrating significant improvements in cutaneous scores and global disease activity (38, 39). In juvenile dermatomyositis, baricitinib has shown efficacy in severe cases complicated by calcinosis and interstitial lung disease, with short-term data indicating enhanced muscle strength and reduced inflammation (40, 41).
4.7. Cutaneous Systemic Sclerosis
Preclinical studies in a bleomycin-induced murine model have demonstrated that selective JAK1/2 inhibition, particularly JAK2 blockade, reduces dermal thickening, collagen deposition, and microvascular loss. Building on this, Hou et al. conducted an open-label pilot trial in 10 patients with diffuse cutaneous systemic sclerosis. Baricitinib 2 mg to 4 mg per day showed a reduction in skin fibrosis and digital ulcers at week 12 and week 24 (42). Another open-label 24-week randomized study (4 mg, 2 mg, control) observed greater improvements in modified Rodnan Skin Score with 4 mg at week 12, with parallel trends in lung function, digital ulcers, and quality of life, without new safety signals (43).
4.8. Anecdotal Efficacy in Other Conditions
Baricitinib has shown promising anecdotal efficacy in treating various refractory inflammatory dermatological conditions. Trachyonychia and other nail changes associated with AA have been reported to improve with baricitinib (44, 45). A phase II trial in 12 patients demonstrated marked efficacy, while case reports highlight benefits in eruptive and nail lichen planus (46-49). Improvement of other inflammatory nail conditions has been reported with baricitinib and other JAK-STAT inhibitors (50). Multiple reports demonstrate efficacy of baricitinib in treatment-resistant pyoderma gangrenosum (51-54). In a retrospective series, 10 months of baricitinib therapy provided significant relief in patients with chronic pruritus of unknown origin, underscoring its therapeutic potential and warranting further randomized, placebo-controlled trials to establish its efficacy and safety profile (55). Refractory generalized granuloma annulare, recalcitrant morphea, extragenital lichen sclerosus, livedoid vasculopathy, cutaneous sarcoidosis, and SAPHO syndrome have significantly improved with baricitinib therapy (56-62). These findings highlight baricitinib’s potential as a versatile treatment for challenging dermatoses, but controlled trials are needed to validate efficacy and safety (Figure 2).
5. Baricitinib in the Pediatric Population
Although baricitinib has gained FDA approval for several adult indications, there remains a significant knowledge gap in its pediatric use. Baricitinib is not FDA approved for use in children under 18 years of age for most conditions, and adult efficacy and safety data cannot be extrapolated to pediatric populations due to key physiological and developmental differences in drug metabolism, immune response, and disease manifestation. The BREEZE-AD-PEDS extension (n = 467) adds meaningful pediatric data in AD, showing sustained efficacy over 52 weeks with predominantly mild adverse events and no new safety signals (27). Complementary signals from treatment refractory juvenile dermatomyositis using weight- and renal-adjusted 4 - 8 mg per day show early cutaneous and muscle improvement and enable corticosteroid tapering without short term serious safety concerns (40). Nonetheless, these findings are indication specific, largely uncontrolled or extension in design, and do not define age-stratified pharmacokinetic/pharmacodynamic (PK/PD) or rare-event risks. Large, randomized pediatric trials with formal PK/PD programs are still required to establish optimized weight-based dosing, durability of benefit, and comprehensive safety needed for broader pediatric use.
6. Baricitinib in Pregnancy and Lactation
Safety data on baricitinib use in pregnancy are limited, with animal studies indicating potential embryotoxic effects; therefore, its use is not advised during pregnancy. The drug should be discontinued at least one month before conception. Women of childbearing age are advised to use contraception during therapy and for one week after the last dose. As baricitinib is excreted in breast milk, breastfeeding should be avoided during treatment and for four days afterward. Prescribing in pregnancy or breastfeeding should be reserved for situations where maternal benefit clearly outweighs fetal or infant risk (4).
7. Adverse Effects and Contraindications
European guidelines recommend a 50% dose reduction in patients older than 75 years, those with recurrent infections, moderate renal impairment (creatinine clearance 30 - 60 mL/min), or concomitant strong organic anion transporter 3 (OAT3) inhibitor use (e.g., probenecid) (4). Baricitinib commonly induces an early, modest reduction in neutrophil count, which stabilizes within normal limits and rarely coincides with severe infection. A transient increase in lymphocyte and platelet counts, as well as a minor, reversible drop in hemoglobin, may occur but generally do not necessitate drug discontinuation (22). Its use is considered safe in mild to moderate hepatic impairment, but is not advised in severe liver disease (63). Cardiovascular adverse events are more frequent in individuals with hypertension or a smoking history (64). Major adverse cardiovascular events (MACE), malignancy, venous thromboembolism (VTE), serious infection, and mortality were low in low risk patients across rheumatoid arthritis, AD, and AA cohorts, and remained low even in at-risk dermatology populations. The incidence rates for MACE, VTE, malignancy, serious infections, and mortality were numerically higher in high risk rheumatoid arthritis but still low overall, supporting individualized risk-benefit assessment (65) (Box 2).
| Variables |
| Common |
| Clinical |
| Upper respiratory infection |
| Headache, nausea |
| Nasopharyngitis |
| Laboratory |
| Neutropenia |
| Lymphopenia |
| Anaemia |
| Raised creatinine levels |
| Raised lipid parameters (hypercholesterolemia, hypertriglyceridemia |
| Transaminitis and raised bilirubin level |
| Raised creatine phosphokinases |
| Thrombocytosis |
| Uncommon |
| Infections: HSV, HZV reactivation, gastroenteritis, and urinary tract infections |
| Arteriovenous thromboembolic events such as pulmonary embolism, and deep vein thrombosis. |
| Myocardial infarction |
| Increased risk of malignancy (lymphoma and non-melanoma skin cancers) |
| Acne |
| Weight gain |
| Contraindications |
| History of hypersensitivity to upadacitinib |
| Pregnancy and breastfeeding; Children < 12 years or < 40 kg |
| Hematologic thresholds |
| Hb < 8g/dL |
| Absolute lymphocyte count < 0.500 cells/mm3 |
| Absolute neutrophil count < 1,000 cells/mm3 |
| Severe hepatic impairment: Child-Pugh C |
| Severe renal impairment: GFR < 15 mL/min/1.73 m2 |
Abbreviations: HSV, herpes simplex infection; HZV, herpes zoster virus.
High-risk population: At least one of the following: Aged 65 years or older, atherosclerotic cardiovascular disease, diabetes mellitus, hypertension, current smoking, HDL cholesterol < 40 mg/dL, Body Mass Index ≥ 30 kg/m2, poor mobility on EQ-5D, or history of malignancy.
8. Monitoring Guidelines
8.1. Baricitinib and Immunizations
Patients taking baricitinib should avoid live vaccines (e.g., rubella, polio, yellow fever), whereas inactivated or heat-killed vaccines (e.g., COVID-19, pneumococcal, influenza vaccines) are safe (Table 1).
| Tests | Timing/Frequency | Special Instructions/Notes |
|---|---|---|
| Complete blood count | Baseline; 1 month after treatment; every 3 - 6 months thereafter | Monitor for cytopenias |
| Lipid profile | Baseline; 12 weeks after treatment initiation | Repeat periodically if elevated; manage per lipid guidelines |
| Liver function test | Baseline | Stop drug if drug-induced liver injury is suspected until excluded |
| Renal function test | Baseline | Discontinue drug if creatinine clearance is 30 - 60 or < 30 mL/min |
| Tb evaluation | Baseline; then annually | QuantiFERON-Gold test or T-SPOT, chest X-ray screen for active and latent TB; treat prior to therapy if positive |
| Viral hepatitis screening | Baseline | Hepatis B: HBsAg, anti-HBs, and total anti-HBc (the triple panel); Hepatitis C: Anti-HCV, antibody contraindicated in active hepatitis B or C infection |
Abbreviations: Tb, tuberculosis; HBsAg, hepatitis B surface antigen; anti-HBs, hepatitis B surface antibody; total anti-HBc, total hepatitis B core antibody; anti-HCV, hepatitis C virus antibody.
8.2. Future Scope
Baricitinib can be considered as a potential alternative choice for moderate-to-severe, recalcitrant AD requiring systemic therapy. It has antifibrotic activity and may serve as an alternative therapy for skin tightening and microvascular manifestations in patients with diffuse cutaneous systemic sclerosis. Its success in treating resistant inflammatory disorders justifies initiating clinical trials of baricitinib for refractory lichen planus and pyoderma gangrenosum. Additionally, topical delivery strategies should be explored for AA, AD, and psoriasis, leveraging localized JAK1/2 inhibition to enhance efficacy while minimizing systemic exposure.
9. Conclusions
In conclusion, baricitinib is well established for the treatment of AD and AA, with this review highlighting robust efficacy observed in these indications and unmet evidence needs in the pediatric population. Although efficacy in psoriasis and SLE appears less promising, vitiligo shows encouraging adjunctive benefits with narrow band-ultraviolet B, warranting adequately powered, long-term, pediatric-inclusive randomized trials.