1. Background
Alopecia areata (AA) is classified as an autoimmune disorder characterized by transient hair loss without lasting scars (1). Approximately 2% of the global population is affected by AA. The incidence of AA is greater in children than in adults and has been noted to be increasing over time. The prevalence of AA varies markedly across different locations (2). The precise etiology of AA remains incompletely elucidated; the dominant theory posits that it involves disruption of immune tolerance within hair follicles due to immunological mechanisms. The progression of AA is influenced by genetic and environmental factors (3). The progression of AA is related to the mobilization and activation of both innate and internal regions of hair follicles, leading to impairment of hair follicle function (4).
Alopecia areata is often diagnosed through the assessment of the clinical presentation of the affected region. In doubtful instances, trichoscopy may serve as a beneficial non-invasive tool to aid in diagnosis, thus circumventing the necessity for invasive procedures such as biopsies (5). The predominant clinical sign of AA is the occurrence of one or more patches. These patches generally display a round or elliptical form and are entirely devoid of hair, resulting in a smooth and hairless appearance (6). Dermoscopy is an invaluable instrument for diagnosing and following up on AA. The predominant trichoscopic features of AA include yellow dots, broken hairs, and micro exclamation mark hairs (7).
The therapeutic approach to AA is influenced by several factors, including the patient’s age, as well as the severity and duration of scalp lesions. Possible therapies include the use of steroids, anthralin, and immunosuppressive medications. It is essential to customize the treatment approach for each case, taking into account their unique needs and circumstances (8). Calcipotriol, a vitamin D analog, regulates the proliferation and development of keratinocytes. It has the capacity to stimulate hair regeneration in AA lesions by affecting the development of B lymphocytes, T lymphocytes, and dendritic cells. Despite being limited, some studies have evaluated the effects of calcipotriol in AA (9).
Platelet-rich plasma (PRP) is an autologous plasma preparation enriched with concentrated platelets, which are abundant in growth factors and cytokines. These components may enhance and facilitate the body’s intrinsic repair and regeneration mechanisms of hair follicles (10). Autologous PRP injections have been documented as useful in treating AA (11).
2. Objectives
The present study sought to evaluate the effectiveness of topical calcipotriol, intralesional autologous PRP injection, and the combination of both treatments in individuals with AA.
3. Methods
3.1. Study Design
This blinded randomized clinical trial was conducted from July 1, 2023, to October 15, 2023, at the Department of Dermatology, Venereology, and Andrology, Faculty of Medicine, Aswan University, Egypt.
3.2. Classification and Grouping of Participants
The research comprised 78 patients, selected from outpatient clinics of the aforementioned department at Aswan University Hospitals, Egypt. Patients’ ages ranged from 5 to 45 years, including 40 females and 38 males.
3.3. Exclusion Criteria
Patients with the following criteria were excluded: Pregnant and breastfeeding females, individuals with compromised immune systems, patients who had undergone systemic management for AA in the previous 3 months, those currently experiencing scalp inflammation, and those who had applied any intradermal or topical therapies within the past four weeks.
3.4. Process
Patients underwent: (1) Full history taking, including occupation, age, course, previous treatment, duration, and systemic illness; (2) full dermatological and general examination to exclude systemic or related autoimmune illnesses; (3) evaluation of the severity of AA using the Severity of Alopecia Tool (SALT) score (12) before treatment and three months following management.
3.4.1. Dermoscopic Examination
Cases were examined using hair dermoscopy (trichoscopy) before and after management by the same examiner with a DL1 dermoscope (DermLite, 3Gen LLC, San Juan Capistrano, CA, USA) (magnification 100X).
3.4.2. Medical Photography
A Canon EOS 1300D 18-megapixel high-resolution digital camera manufactured in Taiwan was used to capture images of the cases. Photographs were taken in a controlled environment, with standardized distance and light, both before and after a 3-month regimen of consistent therapy.
3.5. Treatment Protocol
Patients were randomly divided into three groups using opaque envelopes labeled with unique random codes. Due to the nature of interventions (topical versus intralesional), patients and the treating investigator were not blinded to the received treatment. The evaluations of the outcomes were carried out by two skilled dermatologists who were blinded to the type of treatment received.
- Group I (n = 26): Patients received topical vitamin D3 analog (calcipotriol), 0.005% twice daily for three months.
- Group II (n = 26): Patients received intralesional injections of PRP for six consecutive sessions, two weeks apart.
- Group III (n = 26): Patients received combined topical vitamin D3 analog (calcipotriol), 0.005% twice daily for three months and received intralesional injections of PRP for six consecutive sessions two weeks apart.
3.6. Platelet-Rich Plasma Preparation
The PRP was generated through the standardized double-spin method (150 g for 10 minutes, then 1500 - 2000 g for 10 minutes), yielding approximately 4 - 6 × platelet concentration. No platelet quantification was performed due to limited facilities, which involved drawing 15 mL of venous blood from cases using a 21-gauge butterfly syringe. Blood specimens were collected and transferred to five sterile containers, each containing a 3.8% sodium citrate solution as an anticoagulant. The plasma was effectively separated from red blood cells by centrifuging the sample at 150 g for 10 minutes at ambient temperature. The buffy coat, composed of WBCs and platelets, was present in plasma. Plasma was carefully extracted from a syringe and subsequently transferred to an alternative vessel. It was subjected to an additional centrifugation procedure at ambient temperature for a duration of 10 minutes, with a force of 1500 to 2000 g.
3.7. Platelet-Rich Plasma Injection Technique
Intradermal injections of PRP were administered using aseptic procedures with a 1-mL syringe and 30-gauge needles. Following the application of local anesthetic, an injection of about 0.1 mL of PRP was delivered into the sub-follicular plane of the scalp, maintaining a distance of 1 cm between each injection location. Injection sites on the scalp included the vertex, frontal, and parietal regions.
3.8. Follow-up
The SALT score was used to assess the degree of AA during the initial session and after three months of treatment. Dermoscopic evaluation was conducted before and following three months of treatment. Medical photography was conducted before and after three months of treatment.
3.9. Ethical Considerations
This study was approved by the Institutional Research Ethics Committee at the Faculty of Medicine, Aswan University, Egypt, with the approval number (5834/1/22). All procedures were conducted in accordance with the Declaration of Helsinki (2013 revision). Written informed consent was obtained from all adult participants and from parents or legal guardians of minors. Participant confidentiality and data privacy were strictly maintained. Consent for publication of anonymized clinical data and photographs was obtained. Also clinical trial registration code is NCT05954104.
3.10. Sample Size Calculation
Sample size was determined using G*Power 3 software (13), with a power of 80% and a type I error of 5% (α = 0.05 and β = 80%) on a two-tailed test. The minimum required sample was 75 participants divided into three equal groups: Group I, AA treated with calcipotriol (n = 25); group II, AA treated with PRP (n = 25); and group III, AA treated with combined therapy (n = 25).
3.11. Statistical Analysis
SPSS software version 22 was employed to analyze data. The chi-square test was implemented to compare qualitative variables, resulting in information that included recorded frequencies and percentages. To evaluate a quantitative variable represented by means and SD, Student’s t-test was implemented in statistical analysis. Statistically significant results were related to a P-value < 0.05.
4. Results
The demographic and clinical characteristics of the examined groups are summarized in Table 1. The effect of various management modalities on the SALT score is illustrated in Table 2. Prior to management, an insignificant variance was found in the mean SALT score (P-value = 0.743) among the groups. Group III had an insignificantly greater SALT score (7.8 ± 0.8) compared with group I (6.9 ± 0.5, P-value = 0.597) and group II (6.7 ± 0.9, P = 0.457). There was an insignificant variance between group I and group II (P-value = 0.829) (Figures 1 - 3).
| Parameters | Group I (No. = 26) | Group II (No. = 26) | Group III (No. = 26) | P-Value |
|---|---|---|---|---|
| Age (y) | 18.38 ± 3.3 | 21.85 ± 3.4 | 19.01 ± 3.5 | 0.748 |
| Sex | 0.699 | |||
| Women | 14 (53.8) | 14 (53.8) | 12 (46.2) | |
| Men | 12 (46.2) | 12 (46.2) | 14 (53.8) | |
| BMI | 20.66 ± 4.6 | 21.75 ± 4.2 | 20.11 ± 4.7 | 0.746 |
| Pattern | 1.000 | |||
| Ophiasis | 0 (0) | 0 (0) | 2 (7.7) | |
| Patchy | 26 (100) | 26 (100) | 24 (92.3) | |
| Site-1 | 1.000 | |||
| Beard/scalp | 0 (0) | 0 (0) | 2 (7.7) | |
| Scalp | 26 (100) | 26 (100) | 24 (92.3) | |
| Site-2 | ||||
| Vertex | 16 (61.5) | 14 (53.8) | 14 (53.8) | 0.924 |
| Left profile | 4 (23.1) | 2 (7.7) | 8 (30.8) | 0.341 |
| Right profile | 4 (15.4) | 2 (7.7) | 6 (23.1) | 0.388 |
| Posterior | 2 (7.7) | 8 (30.8) | 14 (58.3) | 0.007 |
| Multiple | 0 (0) | 0 (0) | 4 (16.7) | 0.095 |
| Lesion size (cm2) | 2.35 ± 0.5 | 3.08 ± 0.9 | 7.01 ± 1.4 | 0.001 |
| Number of patches | 1.31 ± 0.6 | 1.08 ± 0.3 | 3.5 ± 0.9 | 0.002 |
a Values are expressed as No. (%) or mean ± SD.
| Parameters | Group I (No. = 26) | Group II (No. = 26) | Group III (No. = 26) | P-Value |
|---|---|---|---|---|
| Baseline | 0.743 | |||
| Mean ± SD | 6.89 ± 0.5 | 6.65 ± 0.9 | 7.78 ± 0.8 | |
| Median (range) | 6.5 (3.6 - 10.5) | 6 (4 - 15) | 6 (3 - 12) | |
| P-value | 1 vs. 2 = 0.829 | 2 vs. 3 = 0.457 | 1 vs. 3 = 0.597 | |
| After three-months | 0.847 | |||
| Mean ± SD | 0.21 ± 0.1 | 0.21 ± 0.1 | 0.39 ± 0.2 | |
| Median (range) | 0 (0 - 1.5) | 0 (0 - 1.5) | 0 (0 - 5) | |
| P-value | 1 vs. 2 = 1.000 | 2 vs. 3 = 0.620 | 1 vs. 3 = 0.620 | |
| P-value | < 0.001 | < 0.001 | < 0.001 | 0.001 |
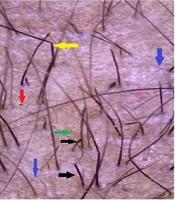
Female patient 9 years old with scalp alopecia areata (AA). A, before treatment [Severity of Alopecia Tool Score (SALT) = 6.3]; B, after 3 months of treatment of topical calcipotriol (SALT = 0.6) (group I); C, dermoscopic picture before treatment (100X): Red arrow, black dots; green arrow, yellow dots; and blue arrows, short vellus hairs; D, dermoscopic picture after treatment (100X).
A 22-year-old male patient with scalp alopecia areata (AA). A, before treatment [Severity of Alopecia Tool Score (SALT) = 6.5]; B, after 3 months of treatment with platelet-rich plasma (PRP) (SALT = 0.7) (group II); C, dermoscopic picture (100X) before treatment: Red arrow, black dot; green arrow, yellow dot; blue arrow, short vellus; black arrow, micro exclamation mark hair; brown arrow, Pohl-Pinkus sign; and yellow arrow, coudability hair; D, dermoscopic picture after treatment (100X).
A 17-year-old male patient with scalp alopecia areata (AA). A and B, before treatment [Severity of Alopecia Tool Score (SALT) = 6.1]; C and D, after 3 months of treatment with a combination of topical calcipotriol and platelet-rich plasma (PRP) (SALT = 0) (group III); E and F, dermoscopic picture before treatment (100X): Red arrows, black dots; green arrows, yellow dots; blue arrow, short vellus hairs; black arrows, micro exclamation mark hairs; G, dermoscopic picture after treatment (100X).
Following three months of treatment, a substantial decline in the mean SALT score was detected across all three groups (P-value = 0.001). The absolute reductions were 3.73 points for group I, 6.87 points for group II, and 18.98 points for group III. The decline was markedly more pronounced in group III (P-value = 0.001), followed by group II, and then group I (Figures 1 - 3).
This study identified side effects of calcipotriol in four participants (7.69%). The adverse effects included erythema, localized discomfort, and a burning sensation. Table 3 illustrates the disparity in trichoscopy results among the examined groups. A substantial drop (P-value = 0.001) in the incidence of yellow dots and exclamation marks was observed across all three groups (Figures 1 - 3).
| Parameters | Group I (No = 26) | Group II (No = 26) | Group III (No = 26) | P-Value |
|---|---|---|---|---|
| Yellow dot | ||||
| Baseline | 18 (69.2) | 20 (76.9) | 22 (84.6) | 0.243 |
| After three-months | 2 (7.7) | 2 (7.7) | 0 (0) | 0.341 |
| P-value | < 0.001 | < 0.001 | < 0.001 | |
| Black dot | ||||
| Baseline | 26 (100) | 20 (76.9) | 26 (100) | 0.621 |
| After three-months | 2 (7.7) | 0 (0) | 2 (7.7) | 0.673 |
| P-value | < 0.001 | < 0.001 | < 0.001 | |
| Exclamation mark | ||||
| Baseline | 18 (69.2) | 16 (61.5) | 22 (84.6) | 0.244 |
| After 3-months | 0 (0) | 0 (0) | 0 (0) | - |
| P-value | < 0.001 | < 0.001 | < 0.001 | |
| Pig tail | ||||
| Baseline | 12 (46.2) | 8 (30.8) | 10 (38.5) | 0.438 |
| After 3-months | 0 (0) | 0 (0) | 0 (0) | - |
| P-value | < 0.001 | < 0.001 | < 0.001 | |
| Vellus hair | ||||
| Baseline | 24 (92.3) | 22 (84.6) | 18 (69.2) | 0.109 |
| After 3-months | 8 (30.8) | 2 (7.7) | 2 (7.7) | 0.086 |
| P-value | < 0.001 | < 0.001 | < 0.001 | |
| Countability hair | ||||
| Baseline | 16 (61.5) | 16 (61.5) | 14 (53.8) | 0.899 |
| After 3-months | 0 (0) | 0 (0) | 0 (0) | - |
| P-value | < 0.001 | < 0.001 | < 0.001 |
a Values are expressed as No. (%).
5. Discussion
Alopecia areata is an autoimmune condition that disrupts the immune privilege of hair follicles. The resulting hair loss is facilitated by the invasion of CD4+ and CD8+ T cells and the secretion of Th1 cytokines, which occur due to disruption of immune privilege in hair follicles (6). The present study revealed a notable reduction in SALT score following a 3-month treatment with topical calcipotriol. This outcome aligns with the findings of Narang et al., Molinelli et al., and Abd-ElRaheem et al., who reported that calcipotriol usage led to expedited hair regrowth and a higher percentage of patches demonstrating a complete response (9, 14, 15). In contrast, Cerman et al. observed that the effectiveness of topical calcipotriol in AA is weak, with an average of 27% total hair regrowth (16). The obtained results can be attributed to the biological actions of vitamin D3 derivatives, which include regulation of proliferation and modulation of cytokine production (17).
Similarly, Albalat and Ebrahim, Khan et al., and Balakrishnan et al. observed a swift enhancement following twelve weeks of intralesional PRP treatment (18-20). This result differs from Gupta et al., who indicated that PRP had limited effectiveness in treating chronic AA (21). The disparity in outcomes may stem from the reduced number of PRP sessions in Gupta et al.’s research, which involved only three sessions, whereas our study administered six sessions over three months (21).
The combination of topical calcipotriol and intralesional autologous PRP injections resulted in a notable reduction in SALT scores. This study demonstrated that group III (combined topical calcipotriol and PRP) saw significantly greater decreases compared to group II (PRP), followed by group I (topical calcipotriol).
This study found no significant variations in microscopic findings across the three groups of AA cases. The yellow dots indicator was prevalent in the three groups, with percentages of 69.2%, 76.2%, and 84.6%, respectively. Guttikonda et al. and Chiramel et al. revealed that the yellow dots sign was prevalent in their studies, with occurrences of 88% and 87.5%, respectively (22, 23). Kibar et al. (24) observed a low incidence of yellow dot symptoms. Inui et al. (25) reported an occurrence of yellow dots at 73%; this discrepancy may be attributed to the yellowish skin tone prevalent among Asian cases, which may complicate the visual identification of yellow dots.
This study corroborated the findings of Chiramel et al. (23) and Amer et al. (26), who reported incidences of black dots at 79.5% and 75%, respectively. Conversely, Inui et al. (25) and Bapu et al. (27) observed that black dots were found in 45% and 36%, respectively. The exclamation mark was seen as a pathognomonic indicator in AA (28). The present study revealed that the prevalence of the exclamation mark was 69.2% in group I, 61% in group II, and 84.6% in group III. These findings correspond with Rakowska et al. (29), who stated a frequency of exclamation marks at 68% in their research. In contrast to research by Mane et al. (30), which indicated a lower incidence of the exclamation mark sign at a rate of 12%. Black spots, tapering hairs, and broken hairs exhibited a positive correlation with disease activity. These findings elucidate discrepancies noted across several studies (30).
In the present study, coudability hair was observed at an incidence of 61.5% in group I, 61.5% in group II, and 53.8% in group III. Rudnicka et al. (31) corroborated our results, noting that coudability hair was detected in 42% of the patients. There are several limitations of this study, such as the lack of biomarkers and PRP quantification. Future multicenter studies should extend follow-up to at least 12 months to assess relapse and durability of remission.
5.1. Conclusions
This study concluded that topical calcipotriol, intralesional autologous PRP injection, and combined topical calcipotriol with intralesional autologous PRP injection were significantly effective in reducing SALT scores in patients with AA. This reduction was significantly more evident in the combined topical calcipotriol with intralesional autologous PRP injection group, followed by the intralesional autologous PRP injection group, and then the calcipotriol group. Future trials should include mechanistic biomarkers, long-term follow-up, and factorial or placebo controls.
![Female patient 9 years old with scalp alopecia areata (AA). A, before treatment [Severity of Alopecia Tool Score (SALT) = 6.3]; B, after 3 months of treatment of topical calcipotriol (SALT = 0.6) (group I); C, dermoscopic picture before treatment (100X): Red arrow, black dots; green arrow, yellow dots; and blue arrows, short vellus hairs; D, dermoscopic picture after treatment (100X). Female patient 9 years old with scalp alopecia areata (AA). A, before treatment [Severity of Alopecia Tool Score (SALT) = 6.3]; B, after 3 months of treatment of topical calcipotriol (SALT = 0.6) (group I); C, dermoscopic picture before treatment (100X): Red arrow, black dots; green arrow, yellow dots; and blue arrows, short vellus hairs; D, dermoscopic picture after treatment (100X).](https://services.brieflands.com/cdn/serve/3170f/396f2a7c719c6d88ce58035a5d84700bb0ae68f9/jssc-12-3-167118-g001-preview.webp)
![A 22-year-old male patient with scalp alopecia areata (AA). A, before treatment [Severity of Alopecia Tool Score (SALT) = 6.5]; B, after 3 months of treatment with platelet-rich plasma (PRP) (SALT = 0.7) (group II); C, dermoscopic picture (100X) before treatment: Red arrow, black dot; green arrow, yellow dot; blue arrow, short vellus; black arrow, micro exclamation mark hair; brown arrow, Pohl-Pinkus sign; and yellow arrow, coudability hair; D, dermoscopic picture after treatment (100X). A 22-year-old male patient with scalp alopecia areata (AA). A, before treatment [Severity of Alopecia Tool Score (SALT) = 6.5]; B, after 3 months of treatment with platelet-rich plasma (PRP) (SALT = 0.7) (group II); C, dermoscopic picture (100X) before treatment: Red arrow, black dot; green arrow, yellow dot; blue arrow, short vellus; black arrow, micro exclamation mark hair; brown arrow, Pohl-Pinkus sign; and yellow arrow, coudability hair; D, dermoscopic picture after treatment (100X).](https://services.brieflands.com/cdn/serve/3170f/50098448fbcbc15442519879e36170c612ad20e6/jssc-12-3-167118-g002-preview.webp)
![A 17-year-old male patient with scalp alopecia areata (AA). A and B, before treatment [Severity of Alopecia Tool Score (SALT) = 6.1]; C and D, after 3 months of treatment with a combination of topical calcipotriol and platelet-rich plasma (PRP) (SALT = 0) (group III); E and F, dermoscopic picture before treatment (100X): Red arrows, black dots; green arrows, yellow dots; blue arrow, short vellus hairs; black arrows, micro exclamation mark hairs; G, dermoscopic picture after treatment (100X). A 17-year-old male patient with scalp alopecia areata (AA). A and B, before treatment [Severity of Alopecia Tool Score (SALT) = 6.1]; C and D, after 3 months of treatment with a combination of topical calcipotriol and platelet-rich plasma (PRP) (SALT = 0) (group III); E and F, dermoscopic picture before treatment (100X): Red arrows, black dots; green arrows, yellow dots; blue arrow, short vellus hairs; black arrows, micro exclamation mark hairs; G, dermoscopic picture after treatment (100X).](https://services.brieflands.com/cdn/serve/3170f/58cee737b0e43eb6b343ec63b837ee4f69e144f1/jssc-12-3-167118-g003-preview.webp)