1. Background
Acne vulgaris (AV) is a chronic, inflammatory disease of the pilosebaceous units. It is the most frequent dermatological disorder, affecting 34.3 - 95% of teenagers and adolescents aged 9 to 18 years old (1). According to El-Hamd et al, the total prevalence of AV was approximately 33.5% among teenagers enrolled in secondary schools in Sohag province, Upper Egypt (2).
The clinical hallmarks of AV include non-inflammatory lesions such as open and closed comedones and inflammatory lesions in the form of papules, pustules, and nodules (3). It affects the skin of visible areas of the body with abundant sebaceous glands, particularly the face and neck, but may additionally occur on the back and chest (1, 4).
The etiopathogenesis of AV is complex, with multiple contributing factors including genetic predisposition, stimulation of androgen hormones, which increases sebum production, changes in lipid composition, follicular hyperkeratinization, bacterial colonization predominantly by Propionibacterium acnes, and periglandular dermal inflammation (5).
The P. acnes triggers inflammation both directly and indirectly; it can activate several pathways which cause the release of inflammatory cytokines [interleukins (IL), such as IL-1, IL-6, IL-8, IL-10, IL-12, and tumor necrosis factor-alpha (TNF-α)], and also activates Toll-like receptors (TLR) such as TLR-2 and TLR-4 through keratinocytes, neutrophils, monocytes/macrophages, natural killer cells, and dendritic cells (including Langerhans cells), resulting in the release of antimicrobial peptides (AMPs, human β defensin 1 and 2) that play an important role in innate immune responses (6, 7).
Many AMPs and human defence proteins (HDPs) are constitutively produced in human skin by keratinocytes, sebocytes, phagocytes, T cells, and mast cells; in contrast, others are induced in response to tissue damage, inflammation, and infection. There is a close association between AMPs/HDPs and the pathogenesis of various skin diseases, including psoriasis, atopic dermatitis, AV, rosacea, systemic lupus erythematosus, systemic sclerosis, and wound healing (8).
Elafin is one of the natural AMPs. It is known as peptidase inhibitor 3 (protein-encoding gene). It was discovered in 1989 to be associated with an inflammatory reaction in the epidermis. It is a member of the whey acidic protein four-disulfide core (WFDC) family and plays a role in regulating inflammation, protecting against tissue damage, and preventing elastase-mediated tissue proteolysis (9, 10).
Elafin is mostly produced constitutively in immune cells, digestive tract, female reproductive tract, lungs, and skin. Its expression is up-regulated by inflammatory stimuli, such as lipopolysaccharide, neutrophil elastase, and pro-inflammatory cytokines like IL-1β or TNF-α (11-13).
2. Objectives
This study aimed to compare serum elafin levels of moderate and severe AV patients versus healthy controls and to assess their levels before and after treatment with systemic isotretinoin.
3. Methods
This case-control study was conducted at the outpatient clinics of Dermatology, Venereology, and Andrology, Faculty of Medicine, Sohag University Hospitals, Sohag, Egypt, from June 2022 to August 2023.
3.1. Ethical Considerations
This study was approved by the Ethical and Scientific Committee at the Faculty of Medicine, Sohag University, Egypt. In addition, informed written consent was obtained from all participants before their examination following the explanation of the nature of the study.
3.2. Sample Size
Sample size calculation was carried out using G*Power 3 software (14). A calculated minimum sample of 60 participants in a 1:1 design [study group (n = 30); included patients with AV and control group (n = 30) included age- and sex-matched healthy controls], was needed to detect an effect size of 0.7 in the mean serum level of elafin concentration, with an error probability of 0.05 and 95% power on a two-tailed test (15).
3.3. Patient Selection
This study included 60 participants, 30 patients of both sexes aged ≥ 12 years with clinically and dermoscopically confirmed moderate and severe AV treated by isotretinoin at a dose of 0.5 - 1 mg/kg per day for 3 months. The controls were 30 completely healthy volunteers (age- and sex-matched).
3.4. Exclusion Criteria
Patients with the following criteria were excluded: patients under 12 years old, smokers, pregnant or lactating women, and women who were not on adequate contraceptive methods, AV patients on current treatment or with a history of topical or systemic treatment of AV for at least 3 months, hepatic dysfunction, renal insufficiency, uncontrolled hyperlipidemia, hypervitaminosis A, myocardial ischemia, inflammatory bowel disease, glomerulonephritis, and rheumatoid arthritis, as well as patients with other dermatological diseases.
3.5. Study Procedures
All patients in this study were subjected to the following:
1. Medical history, including: (A) personal history such as age, sex, marital status, residence, education level, occupation, and special habits; (B) history of acne including onset, course, duration, age of onset, and sites of lesions, exacerbating, and relieving factors; (C) therapeutic history such as previous treatment modalities of AV; (D) family history of AV; (E) past history of chronic medical diseases.
2. General examination was done: (A) to exclude any systemic diseases, e.g., liver disease, renal disease, and hyperlipidemia; (B) to exclude other general causes of elevated serum elafin such as myocardial ischemia, inflammatory bowel disease, glomerulonephritis, and rheumatoid arthritis.
3. Local dermatological evaluation: A local examination of the affected site was done to confirm the diagnosis of AV, detect sites of involvement, and evaluate its severity. The assessment of the severity of acne was performed using the Global Acne Grading System (GAGS) (16).
4. Laboratory investigations: All patients were subjected to laboratory monitoring and evaluations at baseline, after one month of treatment with isotretinoin, and at the end of the 3rd month of treatment by: (A) complete blood picture, liver function tests, renal function tests, lipid profile, and pregnancy test; (B) evaluation of serum level of elafin in all participants; three mL of venous blood was withdrawn from each patient and control under complete aseptic conditions. Acne vulgaris patients' samples were taken before and after 3 months of isotretinoin treatment. Each sample was allowed to clot for 1 hour, the tube was centrifuged for 10 minutes at approximately 3000 × g, and the serum was separated, divided into aliquots, and immediately stored at -80°C until analyzed to determine the serum level of elafin using a Human Elafin (P13) enzyme-linked immunosorbent assay (ELISA) Kit provided by SinoGeneClon Biotech Company. Serum elafin was determined by ELISA according to the manufacturer's protocol for controls and the patients with AV (17).
3.6. Patient Evaluation
Evaluation of patients was done clinically using the GAGS and by photos which were taken using an Android smartphone Samsung Galaxy A21s (model: SM-A217F/DS), rear camera 48 MP wide, 8 MP ultra-wide, 2 MP macro, and 2 MP depth. High-resolution photographs of both sides and the front of the face were taken for all patients with AV at baseline and at the end of the 3rd month of treatment.
3.7. Statistical Analysis
The collected data were verified, coded by the researcher, and analyzed using the Statistical Package for Social Sciences (IBM-SPSS/PC/VER 24). Descriptive statistics: Continuous variables were expressed as mean ± standard deviation, median, range, and qualitative data were expressed as frequencies and percentages. Test of significance: The chi-square/Monte Carlo exact test was used to compare the differences in distribution of frequencies among different groups. The Shapiro-Wilk test was used to test for data normality.
Student t-test and Mann-Whitney U test were calculated to test the mean/median differences in continuous variables between groups (parametric and non-parametric). Multivariate logistic regression analysis was calculated to investigate the independent association between elafin and AV disease [odds ratio (OR), 95% confidence interval (95% CI) and P-value]. The Wilcoxon signed-rank test was used to compare medians between serum elafin levels over time.
A repeated measures ANOVA (RM-ANOVA) test was used to analyze the mean differences in data that followed a normal distribution and had repeated measures. Post-hoc tests were calculated using Bonferroni corrections for pairwise comparisons. The ROC curve was depicted to explore the diagnostic performance of elafin for disease prediction, analyzed as area under the curve (AUC), standard error (SE), and 95% CI. Significant test results were considered when the P-value was < 0.05.
4. Results
There were no statistically significant differences between AV patients and controls in terms of sociodemographic features (Table 1).
| Variables | Patients | Control | P-Value |
|---|---|---|---|
| Age (y) | 18.80 ± 4.1 | 18.43 ± 3.3 | 0.705 b |
| Sex | 0.774 c | ||
| Male | 8 (26.7) | 9 (30) | |
| Female | 22 (73.3) | 21 (70) | |
| Residence | 0.602 c | ||
| Rural | 18 (60) | 16 (53.3) | |
| Urban | 12 (40) | 14 (46.7) | |
| Marital status | 0.554 c | ||
| Single | 29 (96.7) | 28 (93.3) | |
| Married | 1 (3.3) | 2 (6.7) | |
| Educational level | 0.452 c | ||
| Preparatory school | 5 (16.7) | 5 (16.7) | |
| Secondary school | 16 (53.3) | 20 (66.7) | |
| University | 9 (30) | 5 (16.7) | |
| Occupation | 0.153 c | ||
| Student | 20 (66.7) | 19 (63.3) | |
| Graduated | 7 (23.3) | 5 (16.7) | |
| Housewife | 2 (6.7) | 4 (13.3) | |
| Teacher/nurse | 1 (3.3) | 2 (6.7) |
a Values are expressed as No. (%) or mean ± SD.
b Independent sample t-test was used to compare the difference in mean between groups.
c Chi-square test was used to compare the percentages between groups.
Acne vulgaris had a chronic onset in all patients and an intermittent course in 80% of the patients. The mean duration of AV was 5.2 ± 3.3 years. The mean age at onset of AV was 13.6 ± 1.3 years. According to the GAGS Score, 28 (93.3%) patients had moderate AV and 2 (6.7%) patients had moderate/severe AV.
There were statistically significant differences between AV patients and controls regarding the serum level of elafin (P = 0.001; Table 2). There were statistically significant reductions in the GAGS scores and serum elafin levels at the end of the isotretinoin therapy (P < 0.05; Table 3).
Abbreviation: GAGS, Global Acne Grading System.
a Paired Sample t-test was used to compare the difference in mean within group.
b Wilcoxon signed-rank test was used to compare the difference in median within group.
After adjusting for age, sex, and other socio-demographics, serum elafin proved to be a predictor for AV disease; with a 1 ng/mL increase in the serum level of elafin, there was an 11% increase in the risk of having AV (AOR = 1.126, 95% CI: 1.053 - 1.204), and this was statistically significant (P < 0.001; Table 4).
| Factors | OR | 95% CI | LRT P-Value |
|---|---|---|---|
| Age | 0.923 | 0.765 - 1.115 | 0.407 |
| Sex (male vs. female) | 1.337 | 0.308 - 5.803 | 0.698 |
| Residence (urban vs. rural) | 1.312 | 0.472 - 3.653 | 0.603 |
| Educational level | 0.818 | 0.151 - 2.444 | 0.616 |
| Occupation | 1.248 | 0.815 - 1.912 | 0.308 |
| Serum elafin (ng/mL) | 1.126 | 1.053 - 1.204 | < 0.001 |
Abbreviations: OR, odds ratio; CI, confidence interval; LRT, likelihood ratio test.
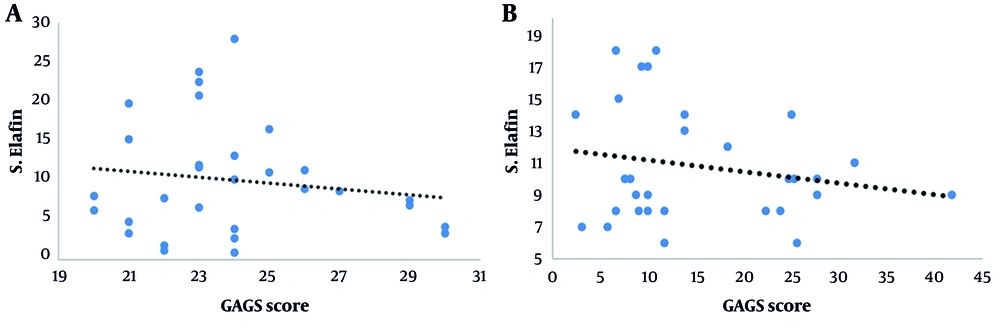
There was a significant negative mild correlation between GAGS score and serum elafin level before treatment (P = 0.042, r = -0.262), and there was a significant negative mild correlation between GAGS score and serum elafin level after treatment (P = 0.048, r = -0.190; Figure 1A and B).
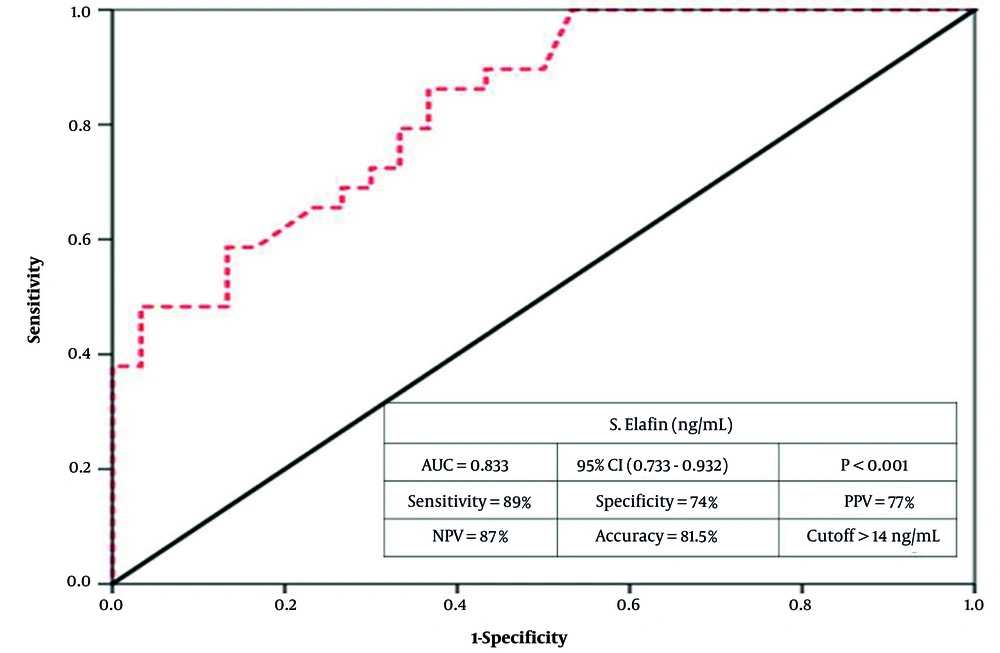
Serum elafin demonstrated excellent validity (AUC = 0.833, P < 0.001; 95% CI: 0.733 - 0.932). Moreover, at a 14 ng/mL cutoff, the validity criteria were as follows: Eighty-nine percent sensitivity, i.e., it correctly identified 89% of AV cases as positive. Additionally, 74% specificity, i.e., it correctly identified 74% of controls as negative. Additionally, the test had 77% precision –– positive predictive value (PPV; the ability of the test to predict AV among all positive cases). It also had 87% negative predictive value (NPV; the ability to predict non-diseased among all those diagnosed as negative). Overall, the test achieved 81.5% accuracy (Figure 2).
5. Discussion
Acne vulgaris is a common chronic inflammatory dermatological disorder of the pilosebaceous unit, with multifactorial etiology and predominantly affects young adults and adolescents (18). Topical antibiotics, retinoids, benzoyl peroxide, alpha hydroxy acids, salicylic acid, or azelaic acid are beneficial in mild cases, while in more severe cases, systemic antibiotics such as tetracycline and doxycycline, oral retinoids, and some hormones are recommended (19).
Elafin is a low molecular weight anti-proteinase that antagonizes human neutrophil elastase, pancreatic elastase, proteinase 3, and endogenous vascular elastase. It also has several functions, including anti-inflammation, immune regulation, anti-microbial, anti-proliferation, vascular remodeling, and wound healing (20).
This study aimed to compare serum elafin levels of moderate and severe AV patients versus healthy controls and to assess their levels before and after treatment with systemic isotretinoin at a dose of 0.5 - 1 mg/kg/day for three months.
In this study, the mean age of the AV group (± SD) was 18.80 ± 4.1 years, and of the control group was 18.43 ± 3.3 years. About 73.3% of AV cases were female. This is similar to the results of a case-control study performed by Ebrahim et al. (21) in Benha University Hospitals, Egypt, as they found that the mean age (± SD) was 20.2 ± 4.44 years in AV patients and 22.08 ± 4.12 years in the control group, and 57.5% of cases were females.
This study reported that the mean serum level of elafin was statistically significantly higher in controls than in AV patients (P = 0.001). This is not in agreement with the case-control study by El-Refaei et al. (15) which was performed on 80 participants; (40 patients suffering from moderate and severe acne, 40 healthy individuals as a control group) at Benha Faculty of Medicine, Egypt, as they found that patients showed significantly higher serum elafin levels when compared to the control group (P < 0.001). In this study, the difference in this result may be explained by the early age of onset (13.6 ± 1.3 years) and chronic and persistent inflammation of AV which may be associated with the following mechanisms: (A) consumption and exhaustion of the regulatory mechanisms of elafin biosynthesis; (B) epigenetically down-regulation of the elafin genes; (C) disruption of the feedback loops that normally resolve inflammation.
There were significant negative mild correlations between GAGS scores and serum elafin levels before and after systemic isotretinoin therapy in moderate and severe AV patients. Additionally, there was a statistically significant reduction in GAGS scores at the end of the isotretinoin therapy. This is similar to Turk et al. (22) who performed a study on 30 patients with moderate to severe AV accompanied by atrophic acne scars treated with oral isotretinoin for 3 months at Istanbul Training and Research Hospital. They found a significant reduction in the GAGS scores at the end of the 3rd month of isotretinoin treatment.
The current study demonstrated that in AV patients, there was a statistically significant reduction in serum elafin level at the end of the isotretinoin therapy. This could be explained by the effect of isotretinoin on modulation of immune and inflammatory signaling in AV; it likely reduces the expression of some AMPs, such as elafin.
In this study, serum elafin showed excellent validity (P < 0.001) at a cut-off value of 14 ng/mL; sensitivity was 89%, specificity was 74%, PPV was 77%, NPV was 87%, and accuracy was 81.5%. This is similar to El-Refaei et al. (15) who found that serum elafin had excellent validity (AUC = 0.928, P < 0.001), at a cut-off value of 0.43, sensitivity was 92.5%, specificity was 82.5%, PPV was 84.1%, NPV was 91.7%, and accuracy was 87.5%.
This study has several limitations including the single-center design, modest sample size, and lack of blinding for outcome assessors. Therefore, the current study needs more confirmation on large-scale, case-controlled, multi-center, clinical studies to perfectly assess the serum levels of elafin and the tissue elafin levels in AV patients. Also, further studies are recommended to evaluate serum elafin level in different grades of AV patients and to assess its correlation with response to different topical and systemic AV treatment modalities.
This study concluded that elafin acts as an alarm anti-protease. Its measurement after oral isotretinoin showed that the serum levels of elafin significantly decreased. The serum elafin is an important inflammatory marker in patients with AV and its use is helpful to measure disease severity, activity, and detect response to therapy.