1. Background
Stroke is one of the most common neurological conditions worldwide, with an estimated 93.8 million prevalent cases and 11.9 million new cases annually (1). A recent study assessing stroke burden and its attributable risk factors from 1990 to 2021 reported an increase in the global stroke burden over this period (1). In 2021, stroke had the highest age-standardized disability-adjusted life years (DALYs) among conditions affecting the nervous system (2). Spasticity is a common complication after stroke, characterized by a velocity-dependent increase in muscle tone and exaggerated tendon reflexes resulting from stretch reflex hyperexcitability (3). A systematic review and meta-analysis of 23 studies found that the pooled prevalence of spasticity after stroke was 25.3% and that after the first-ever stroke was 26.7% (4). Spasticity that interferes with function should be treated, as it can otherwise lead to pain, reduced mobility, impaired performance of daily activities, and a lower quality of life. Dry needling (DN) is a therapeutic modality used by physiotherapists to manage various conditions, including muscle spasticity. A systematic review and meta-analysis concluded that DN has a positive effect in reducing spasticity post-stroke (5). Another systematic review reported that DN may have beneficial effects on spasticity, pain, and range of motion (ROM) (6). Previous research has primarily focused on the effects of DN on spastic wrist flexors (7, 8) or general upper limb spasticity (9, 10). Forearm pronators are commonly affected by spasticity after stroke, contributing to difficulties in reaching, feeding, fingernail trimming, and other self-care activities (11). Despite their clinical relevance, the specific effects of DN on the pronator muscles in relation to spasticity, motor recovery, and functional improvement have not yet been investigated.
2. Objectives
The aim of the present study was to evaluate the effects of DN on the spasticity of forearm pronators, ROM, and upper limb function post-stroke.
3. Methods
3.1. Study Design
This study employed a single-group, sham-controlled design (ethics approval: IR.TUMS.FNM.REC.1402.126; registration: IRCT20230721058873N1). Written informed consent was obtained from all participants after a comprehensive explanation of the study procedure.
3.2. Patients
Participants were individuals with hemiplegia resulting from a first-ever stroke, referred from neurology clinics and rehabilitation centers in Tehran, Iran. Inclusion criteria were: (1) Age between 40 and 75 years; (2) stroke onset at least 6 months prior; (3) a Modified Modified Ashworth Scale (MMAS) score ≥ 1 for spasticity of forearm pronators; (4) no prior DN treatment; and (5) no contraindications to DN (e.g., needle phobia, anticoagulant use, infection, bleeding disorders, mental illness). Exclusion criteria were: (1) The presence of new neurological lesions affecting muscle tone; (2) current use of tone-altering medications; (3) a ≥ 10% reduction in supination range on the affected side; and (4) unwillingness to continue participation.
3.3. Outcome Measures
The primary outcome was spasticity severity, assessed using the Persian version of the MMAS. Secondary outcomes were upper limb motor recovery, assessed by the Brunnstrom recovery stage (BRS), active and passive ROM for elbow extension, forearm supination, and wrist extension, and upper limb functional performance in daily activities, assessed by the Chedoke Arm and Hand Activity Inventory-13 (CAHAI-13).
3.4. Measurements
3.4.1. Spasticity
The MMAS is a reliable and valid tool for assessing the severity of spasticity. The Persian version of MMAS has demonstrated excellent interrater and intrarater reliability in stroke patients (12-15). The MMAS grades spasticity on a scale from 0 (no increase in muscle tone) to 4 (limb rigid in flexion or extension). Assessments were performed with the patient relaxed in a supine position. The physiotherapist passively and rapidly moved the limb, performing only a single movement to avoid influencing the severity of spasticity.
3.4.2. Upper Limb Motor Recovery
Upper limb motor recovery was assessed using the BRS, an ordinal and validated measure of post-stroke motor recovery. The scale consists of six stages, ranging from stage 1 (flaccid paralysis) to stage 6 (normal movement) (16, 17).
3.4.3. Range of Motion
A standard 360° plastic goniometer (Baseline Plastic 360°, 12”, Ghamat Pooyan Evaluation Instruments) was used to measure the active and passive ROM of elbow extension, forearm supination, and wrist extension (18).
3.4.4. Upper Limb Function
The original Chedoke Arm and Hand Activity Inventory (CAHAI) was used to assess the affected arm and hand function (19). It consists of 13 functional items, involving both hands, scored from 13 to 91; higher scores indicate greater functional independence (19). The reliability and validity of the CAHAI-13 have been demonstrated (19, 20).
3.5. Intervention
3.5.1. Real Dry Needling
For DN, patients were positioned supine, with shoulders and arms resting at their sides. The elbow and forearm were placed in full extension and maximal possible supination. The pronator teres was needled 1 cm distal to the midpoint of the elbow crease, between the medial epicondyle and the biceps brachii tendon. The DN of the pronator quadratus was performed with the forearm pronated, three finger-widths proximal to the midpoint between the radial and ulnar styloid processes. A 0.25 × 0.30 mm needle (DongBang AcuPrime Ltd, Korea) was used with the fast-in, fast-out technique in a cone pattern for 1 minute at each point (7, 9).
3.5.2. Sham Dry Needling
Patients were positioned identically to those used for the real DN. Blunt-tipped sham needles were applied to the same points and manipulated by the physiotherapist to simulate the DN procedure without penetrating the skin, thereby creating a sensation similar to actual needle insertion.
3.6. Procedure
Patients received both sham DN and DN in two separate phases, one week apart, without being informed which treatment they received. Each phase consisted of three sessions administered on alternate days. The intervention started with three sessions of sham DN of the pronator muscles at the defined points, followed by a one-week washout period, then three sessions of DN at the same sites. Assessments were performed in randomized order at five time points: T0 (pre-sham), T1 (post-sham), T2 (pre-DN), T3 (post-DN), and T4 (one week after the final DN session). Patients were blinded to the type of intervention (sham or real DN) during each phase.
3.7. Sample Size Calculation
The sample size was calculated using G*Power software (version 3.1.9.4), based on a repeated-measures ANOVA (within-subjects), with one group and five assessments. Assuming an alpha of 0.05, a power of 0.80, and an effect size of 0.3, the required sample size was 17. To account for a potential 10% dropout rate, the final sample size was set at 20 participants.
3.8. Statistical Analysis
Data analysis was performed using SPSS version 26. Quantitative variables were summarized with mean, standard deviation (SD), minimum, and maximum values, while qualitative variables were reported as median, interquartile range (IQR), and frequency percentage. Normality was assessed using the Shapiro-Wilk test. For normally distributed data, repeated measures ANOVA was used, applying the Greenhouse-Geisser correction if Mauchly’s test indicated a sphericity violation. Pairwise comparisons employed Bonferroni post hoc tests. Non-normal data were analyzed with the Friedman test. Ordinal variables (MMAS and BRS) were examined using the Wilcoxon Signed-Rank Test. Effect size was calculated using Cohen’s d. Statistical significance was set at P < 0.05.
4. Results
4.1. Participant Characteristics
Of the 22 patients initially assessed, 20 patients (14 males, 6 females) met the inclusion criteria and were enrolled in the study. The mean age was 55.3 ± 9.5 years, and the mean time since stroke onset was 15.5 ± 11.4 months.
4.2. Spasticity Severity
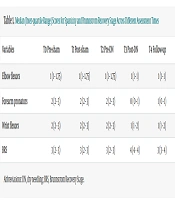
No significant change in median spasticity scores of the elbow flexors was observed across all assessments following sham and DN interventions (χ2 = 8.00, df = 4, P = 0.09); the median score remained at 1 (Table 1).
| Variables | T0 Pre-sham | T1 Post-sham | T2 Pre-DN | T3 Post-DN | T4 Follow-up |
|---|---|---|---|---|---|
| Elbow flexors | 1 (1 - 1.75) | 1 (1 - 1.75) | 1 (1 - 1.75) | 1 (1 - 1) | 1 (1 - 1) |
| Forearm pronators | 2 (2 - 3) | 2 (2 - 3) | 2 (2 - 3) | 0 (0 - 1) | 1 (0 - 1) |
| Wrist flexors | 2 (2 - 3) | 2 (2 - 3) | 2 (2 - 3) | 1 (1 - 2) | 1 (1 - 2) |
| BRS | 3 (2 - 3) | 3 (2 - 3) | 3 (2 - 3) | 4 (4 - 4) | 3 (3 - 4) |
Abbreviations: DN, dry needling; BRS, Brunnstrom Recovery Stage.
The Friedman test showed a significant reduction in forearm pronator spasticity following DN (χ2 = 76.36, df = 4, P < 0.001), with median scores decreasing from 2 (IQR: 2 - 3) at T2 to 0 (0 - 1) at T3 and 1 (0 - 1) at T4. Wilcoxon tests revealed no significant change after sham DN (Z = 1.00, P = 0.31) and no difference between T0 and T2 (Z = 0.00, P = 1.00). In contrast, DN significantly reduced spasticity from 2 at T0 and T2 to 0 at T3 (Z = 4.09, P < 0.001), with no significant difference between T3 and T4 (Z = 1.73, P = 0.08).
The Friedman test revealed a significant reduction in wrist flexor spasticity following DN (χ2 = 57.45, df = 4, P < 0.001), with median scores decreasing from 2 (IQR: 2 - 3) at T2 to 1 (1 - 2) at T3 and T4. Wilcoxon tests showed no significant change after sham DN and no difference between T0 and T2 (Z = 0.00, P = 1.00). However, spasticity significantly declined from 2 at T0 and T2 to 1 at T3 (Z = 3.53, P < 0.001), with no further change between T3 and T4 (Z = 1.00, P = 0.31).
4.3. Brunnstrom Recovery Stage Scores
The Friedman test showed significant changes in median BRS scores across the five time points (χ2 = 52.1, df = 4, P < 0.001; Table 1). Wilcoxon tests indicated no effect of sham DN and no difference between T0 and T2 (Z = 0.00, P = 1.00). In contrast, DN significantly increased scores from 3 (IQR: 2 - 3) at T2 to 4 (4 - 4) at T3 (Z = 3.64, P < 0.001), with T3 also significantly higher than T0 (P < 0.001). A decline to 3 (3 - 4) at T4 was observed (Z = 3.07, P < 0.001), though scores remained significantly improved compared to T0 and T2 (Z = 2.07, P = 0.03).
4.4. Active Range of Motion
Table 2 presents the mean ± SD, and minimum-maximum values for active ROM across the five evaluation time points.
| Variables | T0 Pre-sham | T1 Post-sham | T2 Pre-DN | T3 Post-DN | T4 Follow-up |
|---|---|---|---|---|---|
| Elbow extension | 105.7 ± 24.7 (59 - 135) | 106.4 ± 25.3 (60 - 137) | 105.8 ± 25.1 (58 - 135) | 117.2 ± 19.5 (73 - 137) | 114.2 ± 20.7 (66 - 136) |
| Forearm supination | 76.2 ± 50.8 (0 - 142) | 77.2 ± 51.3 (0 - 143) | 76.4 ± 50.7 (0 - 143) | 106.8 ± 47.0 (15 - 152) | 85.1 ± 55.0 (0 - 148) |
| Wrist extension | 17.4 ± 13.6 (0 - 45) | 17.6 ± 13.8 (0 - 45) | 17.3 ± 13.5 (0 - 45) | 24.7 ± 11.9 (0 - 47) | 23.0 ± 11.8 (0 - 46) |
Abbreviation: DN, dry needling.
a Values are expressed as mean ± standard deviation (SD, minimum – maximum).
4.4.1. Active Elbow Extension
The Friedman test showed significant changes in active elbow extension ROM across five time points (χ2 = 39.13, df = 4, P < 0.001). Sham DN resulted in a small, temporary increase in ROM from 105.7° at T0 to 106.4° at T1 (Z = 2.21, P = 0.02, d = 0.02), which declined to 105.8° at T2 (Z = 2.40, P = 0.01), showing no significant difference from baseline (P = 0.68). In contrast, DN produced a significant improvement, increasing ROM from 105.8° at T2 to 117.2° at T3 (Z = 3.06, P < 0.01, d = 0.5). Although ROM slightly decreased to 114.2° at T4 (Z = 2.67, P < 0.01), it remained significantly higher than at T0 and T2 (P < 0.01). The ROM at T3 was also significantly greater than that at T1 (Z = 2.80, P < 0.01), highlighting the superior effect of DN over sham DN.
4.4.2. Active Forearm Supination
The Friedman test revealed significant differences in active forearm supination ROM across five time points (χ2 = 66.04, df = 4, P < 0.001). Sham DN produced a modest increase in ROM from 76.2° at T0 to 77.2° at T1 (Z = 2.20, P = 0.02, d = 0.02), which then declined to 76.4° at T2 (Z = 2.06, P = 0.03), showing no significant difference from baseline (P = 0.1). In contrast, DN led to a significant improvement, increasing ROM from 76.4° at T2 to 106.8° at T3 (Z = 3.92, d = 0.62, P < 0.001), which was also significantly higher than T0 (P < 0.001). Although ROM declined to 85.1° at T4 (Z = 3.92, P < 0.001), it remained significantly above T0 and T2 levels (P < 0.01). Furthermore, ROM at T3 exceeded that at T1 (Z = 3.92, P < 0.001), confirming the superior effectiveness of DN over sham DN.
4.4.3. Active Wrist Extension
A one-way repeated measures ANOVA revealed significant changes in active wrist extension ROM across five time points (Mauchly’s test: χ2 = 206.67, P < 0.001; Greenhouse-Geisser correction: F(1.09, 20.72) = 18.96, P < 0.001). Bonferroni analysis showed no significant effect of sham DN (P = 1.00), with no difference between T0 and T2 (P = 1.00). In contrast, DN significantly increased ROM from 17.3° at T2 to 24.7° at T3 (P < 0.01, d = 0.57), a change also significant compared to T0 (P < 0.01). Although ROM slightly declined to 23.0° at T4 (t = 3.79, P = 0.01), it remained significantly higher than those at T0 and T2 (P < 0.01), indicating a sustained improvement.
4.5. Passive Range of Motion
Table 3 presents the mean, SD, and minimum-maximum values for passive ROM across the five evaluation time points.
| Variables | T0 Pre-sham | T1 Post-sham | T2 Pre-DN | T3 Post-DN | T4 Follow-up |
|---|---|---|---|---|---|
| Elbow extension | 141.1 ± 4.1 (134 - 148) | 141.5 ± 4.3 (135 - 149) | 141.4 ± 4.0 (135 - 148) | 144.6 ± 3.8 (135 - 150) | 143 ± 3.9 (135 - 150) |
| Forearm supination | 126.2 ± 41.0 (0 - 157) | 126.7 ± 41.3 (0 - 161) | 125.9 ± 41.1 (0 - 158) | 167.2 ± 8.5 (145 - 175) | 164.8 ± 8.3 (145 - 175) |
| Wrist extension | 37.0 ± 19.76 (0 - 56) | 37.1 ± 20.1 (0 - 58) | 36.8 ± 19.8 (0 - 57) | 54.2 ± 6.1 (40 - 61) | 52.5 ± 6.0 (40 - 60) |
Abbreviation: DN, dry needling.
a Values are expressed as mean ± standard deviation (SD, minimum – maximum).
4.5.1. Passive Elbow Extension
A one-way repeated measures ANOVA revealed significant differences in passive elbow extension ROM across five time points (Mauchly’s test: χ2 = 69.07, P < 0.001; Greenhouse-Geisser correction: F(1.58, 30.04) = 14.36, P < 0.001). Bonferroni analysis showed no significant effect of sham DN (P = 0.58) and no change between T0 and T2 (P = 1.00). In contrast, DN significantly increased ROM from 141.4° at T2 to 144.6° at T3 (P < 0.01, d = 0.82), with T3 also significantly higher than T0 (P < 0.01). Although ROM slightly declined to 143° at T4 (P = 0.05), it remained elevated, indicating a sustained improvement.
4.5.2. Passive Forearm Supination
The Friedman test revealed significant differences in passive forearm supination ROM across five time points (χ2 = 73.10, df = 4, P < 0.001). Wilcoxon analysis showed no significant effect of sham DN (Z = 1.89, P = 0.06) and no difference between T0 and T2 (Z = 0.94, P = 0.34). In contrast, DN significantly increased ROM from 125.9° at T2 to 167.2° at T3 (Z = 3.92, P < 0.001, d = 1.38), with the T3 value also significantly higher than at T0 (P < 0.001). Although a slight decrease occurred from T3 to T4 (167.2° to 164.8°; Z = 3.33, P < 0.01), ROM at T4 remained significantly elevated compared to T0 and T2 (P < 0.001), indicating sustained improvement following DN.
4.5.3. Passive Wrist Extension
The Friedman test showed significant differences in passive wrist extension ROM across five time points (χ2 = 53.04, df = 4, P < 0.001). Wilcoxon analysis revealed no significant effect of sham DN (Z = 0.4, P = 0.68) and no difference between T0 and T2 (Z = 0.95, P = 0.34). In contrast, DN significantly increased ROM from 36.8° at T2 to 54.2° at T3 (Z = 3.62, P < 0.001, d = 1.18), with the T3 value also significantly higher than at T0 (P < 0.001). Although ROM slightly declined to 52.5° at T4 (Z = 2.96, P < 0.01), it remained significantly elevated compared to T0 and T2 (P < 0.001), indicating sustained improvement after DN.
4.6. Upper Limb Function (Chedoke Arm and Hand Activity Inventory-13 Scores)
The Friedman test showed a significant difference in CAHAI-13 scores across five time points (χ2 = 37.60, df = 4, P < 0.001). Wilcoxon analysis indicated no significant effect of sham DN (P = 1.00), with no change between T0 and T2 (P = 1.00). In contrast, DN significantly improved scores from 26.5 at T2 to 27.6 at T3 (Z = 2.99, P < 0.01, d = 0.09), with T3 scores also significantly higher than at T0 (P < 0.01). The slight decrease to 27.4 at T4 was not statistically significant (Z = 1.63, P = 0.1), indicating sustained functional improvement.
5. Discussion
This study aimed to evaluate the effect of DN applied to the forearm pronators on spasticity and upper limb function in patients post-stroke. The results demonstrated that DN significantly improved all measured outcomes, except for elbow flexor spasticity.
5.1. Spasticity Severity
The results of this study demonstrated that DN of the affected forearm pronators produced a clinically meaningful reduction in spasticity, evidenced by a 2-point decrease in the MMAS score for the forearm pronators and a 1-point decrease for the wrist flexors. These changes exceeded the minimal clinically important difference (MCID = 0.48) (21) and were sustained for up to one week after the last DN session. Furthermore, sham DN had no significant effect on spasticity severity. The observed improvements in spasticity are consistent with previous studies reporting the beneficial effects of DN on post-stroke spasticity (5-10).
In this study, no change in elbow flexor spasticity was observed after DN. One possible explanation is the low baseline severity of elbow flexor spasticity, which remained at grade 1 both before and after the intervention. In contrast, a recent study evaluating the immediate effects of DN on spastic muscles in patients with stroke reported a significant reduction in elbow flexor spasticity, with the mean MMAS score decreasing from 1.81 ± 0.84 to 1.25 ± 0.46 (22). This discrepancy may suggest that DN is more effective in cases of moderate to severe spasticity. Another possible explanation is the difference in needling targets: Unlike the study by Friedman et al. (22), in which the elbow flexors were directly needled, our intervention did not involve direct DN of the elbow flexors. Instead, we aimed to assess whether DN applied to the forearm pronator muscles could produce indirect effects on other spastic muscles. The lack of significant improvement in elbow flexor spasticity may thus reflect the importance of targeting specific muscles directly when using DN to reduce spasticity.
Nevertheless, the present study demonstrated that, in addition to reducing spasticity in the pronator muscles, DN also significantly decreased spasticity in the wrist flexors. This broader effect may be attributed to the disruption of abnormal spasticity patterns within synergistic muscle groups, potentially improving overall motor control and facilitating more selective muscle activation. Given that the pronator quadratus muscle has not been specifically targeted in previous DN studies, comparing the effects of DN on the pronator teres versus the pronator quadratus may provide valuable insights into spasticity management and upper limb function. Investigating potential differences in their response to DN may deepen our understanding of its mechanisms and therapeutic effectiveness, while also highlighting the often-overlooked role of the pronator quadratus in rehabilitation and motor recovery.
5.2. Passive Range of Motion
Improving passive ROM is important to maintain and increase joint mobility, to prevent the development of fixed contractures, and to enhance the potential for active movement. Spastic muscles can restrict passive movements, and therefore improvement in passive ROM is a critical measure of progress in stroke patients with spasticity. The results of this study demonstrated that DN of the affected pronator muscles significantly increased the passive ROM in elbow extension, forearm supination, and wrist extension (large effect size: d > 0.8). These improvements were maintained up to one week after the third DN session. In contrast, sham DN had no effect on passive ROM in any joint. These findings align with previous studies, either case reports or group studies performed on the spastic upper limb (7-9, 23-25). A systematic review of sixteen studies concluded that DN, either used as a single intervention or combined with other therapies, has beneficial effects in reducing spasticity and improving ROM in stroke patients (6). The small gains in passive elbow extension observed in this study may be attributed to participants already having near-normal extension ROM at baseline (~ 141°). The increase in passive ROM can be attributed to a reduction in muscle spasticity and mechanical alterations conducive to joint mobility following DN.
5.3. Active Range of Motion
The results of this study showed that DN of the affected pronator muscles significantly increased the mean active ROM in elbow extension, forearm supination, and wrist extension (medium effect size: d = 0.5, 0.62, 0.57, respectively), beyond the effects observed with sham treatment. These findings are consistent with previous studies reporting significant improvements in active ROM following DN, either as a standalone intervention or in combination with exercise therapy, across various study designs (7-9, 26, 27).
It is worth noting that this study specifically targeted only the forearm pronator muscles. Despite this focused approach, DN of pronators led to significant improvements in active wrist and elbow extension ROM, as well as a significant increase in active forearm supination. These results highlight the broader functional benefits of targeting the pronator muscles. However, greater improvements were observed in active and passive ROM in forearm supination. This indicates that to achieve greater effects in spasticity reduction, the specific spastic muscles should be targeted by DN.
The observed improvements in active ROM following DN may be attributed to reductions in spasticity severity, improvement in corticospinal tract integrity (24), functional recovery within the affected primary motor cortex (28), and positive effects on brain network (29).
5.4. Brunnstrom Recovery Stage
The results of this study demonstrated that DN of the affected pronator muscles significantly improved the BRS, whereas sham DN had no effect on this outcome. This improvement is in line with findings from previous works (7, 9, 10, 30). The observed improvement in BRS may be attributed to reductions in spasticity and improvements in active ROM.
5.5. Upper Limbs Function
The CAHAI-13 revealed a statistically significant improvement after DN and maintained one week post-intervention. However, considering that the MCID for the CAHAI-13 is 6.3 points (20), the observed change of 1.1 points in this study, from 26.5 at baseline to 27.6 at the end of the third DN session, was not deemed clinically meaningful. In contrast, sham DN of the same muscles had no significant effect on this outcome. The lack of significant changes in the CAHAI-13 scores could be due to the absence of task-oriented training. Combining DN of the pronator muscles with task-oriented training at an adequate intensity and frequency (8) may lead to greater improvements in upper limb function in patients with stroke. Further research is needed to test this hypothesis using the CAHAI-13 as an outcome measure in larger, controlled studies.
5.6. Conclusions
The DN of the affected forearm pronators in patients with chronic stroke significantly reduced spasticity not only in the needled pronator muscles but also in the wrist flexors. Furthermore, it resulted in significant improvements in both active and passive ROM, specifically in elbow extension, forearm supination, and wrist extension. In addition, upper limb motor recovery was improved, as evidenced by an increase in the BRS score. These promising results warrant further investigation to confirm the findings and examine the long-term effects of DN.
5.7. Study Limitations
This study has several limitations that should be acknowledged. First, we lacked an independent sham control group. Second, the order of administration of real and sham interventions was not randomized. All patients received sham DN first, followed by active DN. We assumed that sham DN would have no effects, and therefore aimed to control for carryover effects. Despite this, we implemented a one-week washout period between sham DN and active DN. Third, although the sample size met our power calculation, the cohort was small, limiting the generalizability of our findings to the broader stroke patient population. Fourth, even with patient blinding, there is a possibility that participants may have guessed the intervention type due to sensations or effects, especially in physical treatments like DN. Fifth, another limitation is the potential bias from the lack of assessor blinding. The outcome assessor and intervention provider were the same, introducing a risk of bias in both measurement and DN delivery.