1. Background
Varicocele is characterized by the abnormal enlargement and twisting of the pampiniform plexus veins within the scrotum, primarily associated with an increase in testicular temperature. It is recognized as a significant cause of male infertility, contributing to impaired sperm production and function. Varicoceles are present in approximately 15% to 20% of the general male population, yet their prevalence rises to about 40% among men experiencing infertility (1). Although the precise mechanisms by which varicoceles affect sperm production, morphology, and function remain unclear, multiple hypotheses have been proposed. The link between clinically significant varicoceles and male infertility is well-established. This correlation was initially observed by Barfield, a British surgeon, in the late 19th century and was later confirmed by subsequent studies in the early 20th century (2).
Male factors play a significant role in the infertility observed among couples struggling to conceive. Infertility affects approximately 15% of the global population. One of the most severe forms of male infertility is azoospermia, which is defined as the complete absence of spermatozoa in two separate centrifuged semen samples. In contrast, aspermia refers to the total absence of ejaculate. Azoospermia is estimated to affect about 1% of all men and is responsible for 10% to 15% of infertility cases among men (3-5).
The exact impact of varicocele on testicular function is still unclear, but several mechanisms have been proposed to explain the resulting testicular dysfunction. These include alterations in the temperature regulation of the pampiniform plexus, the backflow of renal and adrenal metabolites from the renal vein into the internal spermatic vein, and decreased blood flow and stasis in the vessels surrounding the testicle, which can lead to hypoxia (3, 6).
Varicocelectomy, especially via laparoscopic techniques, has been shown to significantly improve semen parameters (7, 8), with meta-analyses supporting the efficacy of all surgical approaches. However, recurrence rates and treatment failures remain challenges, particularly for recurrent varicoceles (9). Importantly, the definition of surgical success varies across studies and may include improvements in semen parameters, reduction in recurrence rates, and/or achieving pregnancy. This study defines success for laparoscopic varicocelectomy as a combination of these outcomes.
Moreover, factors such as patient age, baseline semen quality, duration of infertility, and presence of concurrent female infertility factors can confound surgical outcomes and require consideration in evaluating treatment success. Despite the prevalence of recurrent varicocele and the need for repeat surgery, there is limited research on predictors of success following laparoscopic varicocelectomy for recurrent cases.
2. Objectives
This quasi-experimental study aims to evaluate changes in sperm parameters following laparoscopic varicocelectomy in patients with recurrent varicocele, addressing a gap in current evidence.
3. Methods
3.1. Study Design and Participants
This quasi-experimental study was conducted at Aria Hospital in Ahvaz, Iran, between April 2024 and April 2025. The study included male patients with a history of varicocele surgery who presented with either persistent infertility or recurrent varicocele. Inclusion criteria consisted of male patients with a documented history of at least one prior varicocele surgery, accompanied by clinical and/or ultrasound-confirmed recurrence. Additionally, patients had to report infertility, defined as the inability to achieve pregnancy after at least 12 months of regular unprotected sexual intercourse.
Exclusion criteria included the presence of known genetic causes of azoospermia, such as Klinefelter syndrome; confirmed severe female factor infertility in the couple; and the use of medications or existence of systemic medical conditions that could negatively affect male fertility. A total of 38 patients were enrolled in the study, and no participants were lost to follow-up.
Sample size was calculated using G*Power software, based on a study by Liu et al. (10). Assuming an effect size of 0.5, a confidence interval of 95%, and a test power of 90%, the minimum required sample size was determined to be 38.
The study was approved by the Medical Ethical Committee of Ahvaz Jundishapur University of Medical Sciences (ethics code IR.AJUMS.HGOLESTAN.REC.1403.114).
3.2. Data Collection and Assessment
After obtaining written informed consent, each patient completed a structured questionnaire. Additional clinical data were retrieved from medical records. All semen analyses were performed at a single certified andrology laboratory, both prior to and three months after the surgery. Semen parameters were interpreted based on WHO 2010 reference criteria (11). Quality control measures were implemented, including calibration of the analyzer and staff training. Laboratory personnel were blinded to the clinical status of the patients to minimize bias.
Color Doppler ultrasound examinations were conducted by a single experienced sonographer, measuring testicular volume, venous diameter, and the degree of venous reflux (graded as low, intermediate, or high). All patients underwent laparoscopic varicocelectomy, performed by the same surgical team. Follow-up assessments, including repeat semen analysis and ultrasonography, were carried out three months postoperatively.
Surgical success was defined by improvement in at least one of the following: Semen quality parameters (concentration, motility, and morphology), testicular volume, or reduction in venous diameter or reflux grade on Doppler ultrasound.
3.3. Statistical Analysis
Descriptive statistics were presented as means ± standard error of the mean (SEM) or frequencies and percentages. The Kolmogorov-Smirnov test was used to evaluate the normality of the data distribution. To compare pre- and post-operative semen parameters, the paired -test was applied for normally distributed variables. Pearson’s correlation coefficient was used to assess associations between continuous variables, and Spearman’s rank correlation was used for ordinal or non-parametric variables. Statistical analyses were conducted using SPSS version 23, with P-values < 0.05 considered statistically significant.
4. Results
Baseline demographic, clinical, and hormonal characteristics were recorded before laparoscopic varicocelectomy (Tables 1 and 2). A total of 38 patients with a history of varicocele surgery were included in the study, with no cases of dropout or loss to follow-up. The mean age of participants was 35.53 ± 1.11 years, and the average time since the initial surgery was 4.63 ± 0.42 years. Mean testicular volume was 23.53 ± 0.79 mL on the right and 21.63 ± 0.55 mL on the left. The mean diameter of the testicular veins was 2.32 ± 0.09 mm on the right and 3.09 ± 0.12 mm on the left (Table 1).
| Variables b, c | Right | Left |
|---|---|---|
| Testis volume | 23.53 ± 0.79 | 21.63 ± 0.55 |
| Diameter of testicular veins | 2.32 ± 0.09 | 3.09 ± 0.12 |
| Back flow | ||
| Without Valsalva | 26 (68.42) | 5 (13.15) |
| Low | 10 (26.31) | 23 (60.52) |
| Moderate | 2 (5.27) | 8 (21.05) |
| High | 0 (0.00) | 3 (5.28) |
| With Valsalva | 9 (23.68) | 2 (5.26) |
| Low | 3 (60.52) | 8 (21.05) |
| Moderate | 6 (15.8) | 16 (42.10) |
| High | 0 (0.00) | 12 (31.59) |
a Values are expressed as mean ± standard error of the mean (SEM) for continuous variables and as No. (%) for categorical variables.
b Mean ± SEM of age (y) = 35.53 ± 1.11.
c Mean ± SEM of time elapsed since initial surgery (y) = 4.63 ± 0.42.
| Variables | Values |
|---|---|
| FSH | 2.01 ± 0.20 |
| LH | 4.72 ± 0.24 |
| Testosterone | 1.98 ± 0.16 |
| PRL | 8.69 ± 0.343 |
Abbreviations: FSH, follicle-stimulating hormone; LH, luteinizing hormone; PRL, prolactin.
a Values are expressed as mean ± standard error of the mean (SEM).
Assessment of venous backflow via color Doppler ultrasound revealed varying degrees of reflux. Without Valsalva, low to moderate backflow was observed in 68.4% of patients on the right and 78.9% on the left. With the Valsalva maneuver, increased backflow was seen in 73.7% of cases on both sides. The grading of backflow was based on the duration of reflux: Low (< 1 second), moderate (1 - 2 seconds), and high (> 2 seconds). These classifications were determined according to Doppler findings during and without Valsalva (Table 1).
Hormone levels were recorded as follows: Follicle-stimulating hormone (FSH) was 2.01 ± 0.20, LH was 4.72 ± 0.24, testosterone was 1.98 ± 0.16, and PRL was 8.69 ± 0.34 (Table 2).
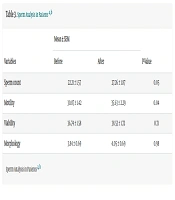
Sperm analysis was performed manually before and three months after laparoscopic varicocelectomy. A paired t-test showed a statistically significant increase in sperm count from 22.21 ± 1.57 to 27.26 ± 1.87 million/mL (P = 0.05), and in motility from 30.87 ± 1.42% to 35.63 ± 2.29% (P = 0.04). No significant differences were observed in viability (36.74% vs. 38.53%, P = 0.71) or morphology (3.84% vs. 4.05%, P = 0.98; Table 3).
| Variables | Mean ± SEM | P-Value | |
|---|---|---|---|
| Before | After | ||
| Sperm count | 22.21 ± 1.57 | 27.26 ± 1.87 | 0.05 |
| Motility | 30.87 ± 1.42 | 35.63 ± 2.29 | 0.04 |
| Viability | 36.74 ± 1.58 | 38.53 ± 1.72 | 0.71 |
| Morphology | 3.84 ± 0.69 | 4.05 ± 0.69 | 0.98 |
Abbreviation: SEM, standard error of the mean.
a Differences between pre- and post-operative values were analyzed using the paired t-test.
b P-values < 0.05 were considered statistically significant.
Pearson correlation showed that post-surgical left testicular volume had a moderate, statistically significant positive correlation with sperm motility (r = 0.58, P < 0.001). Other sperm parameters showed no significant association with testicular volume (Table 4).
a Pearson’s correlation coefficient was used to assess relationships between continuous variables.
b r = Correlation coefficient.
c R2 = Coefficient of determination.
The diameter of testicular veins on both sides was significantly associated with sperm count (right r = 0.51, P = 0.02; left r = 0.55, P = 0.01) and motility (right r = 0.46, P = 0.04; left r = 0.66, P < 0.001). No significant associations were found with viability or morphology (Table 5).
a Pearson’s correlation coefficient was applied.
b P-values < 0.05 were considered statistically significant.
c r = Correlation coefficient.
d R2 = Coefficient of determination.
Spearman correlation analysis revealed that greater backflow with Valsalva, particularly on the left side, was negatively correlated with sperm count (r = 0.58, P < 0.001) and motility (r = 0.49, P = 0.03). A significant inverse correlation was also found between backflow with Valsalva on the left and morphology (r = 0.58, P = 0.00). Viability was not significantly correlated with any measure of backflow (Table 6).
| Variables | Correlation/Spearman r (Backflow) | |||||||
|---|---|---|---|---|---|---|---|---|
| Right | Left | |||||||
| With Valsalva Maneuver | Without Valsalva Maneuver | With Valsalva Maneuver | Without Valsalva Maneuver | |||||
| r b | P-Value | r b | P-Value | r b | P-Value | r b | P-Value | |
| Sperm count | 051 | 0.02 | 0.37 | 0.13 | 0.58 | 0.00 | 0.48 | 0.03 |
| Motility | 0.48 | 0.03 | 0.29 | 0.22 | 0.42 | 0.06 | 0.49 | 0.03 |
| Viability | -0.10 | 0.65 | -0.09 | 0.70 | 0.11 | 0.64 | -0.16 | 0.49 |
| Morphology | 0.12 | 0.62 | 0.40 | 0.08 | 0.54 | 0.01 | 0.58 | 0.00 |
a Spearman’s rank correlation was used to evaluate the relationship between sperm parameters and backflow grades.
b r = Spearman correlation coefficient.
5. Discussion
Varicocele impairs Leydig cell function in both testes, leading to decreased intratesticular testosterone levels. This reduction affects Sertoli cell function, disrupting spermatogenesis and sperm maturation in the epididymis. Testicular testosterone binds to Sertoli cell proteins rather than liver globulins. Consequently, varicocele results in reduced acrosin levels, increased oxidative stress, and sperm DNA damage, contributing to decreased fertility in men (12, 13).
Some observations about varicocele are not yet fully understood. Interestingly, some patients with varicocele exhibit one, two, or three abnormal parameters in their semen analysis and are infertile, while a significant number of men with varicocele have normal sperm parameters and remain fertile (12, 14). To clarify this conflicting situation, Harrison et al. suggested that testicular complications arising from left-sided varicocele may be due to increased extracellular fluid in the testicles (known as testicular extracellular edema). Thus, if men with left varicocele have an efficient testicular lymphatic drainage system, they are less likely to experience extracellular testicular edema, which would mean their testicular function remains intact. On the other hand, it can be speculated that the fertility potential of some men with varicocele who have successfully fathered children might be temporary. As they age, they may become infertile without being aware of it (15).
The question of whether treating varicocele improves semen quality or fertility potential in men has yielded varied results across different studies. For instance, one study observed no significant improvement in sperm parameters, such as morphology and progressive motility, in men who underwent surgery compared to those who remained untreated over a follow-up period of 53 months (16). Conversely, other studies have indicated that treatment for varicocele in infertile men can lead to improvements in sperm parameters and increased fertility rates (2, 17-20).
In our study, we found that sperm viability and morphology did not show any significant changes before and after varicocelectomy. However, sperm count and motility were significantly higher following the surgical intervention compared to the measurements taken before the procedure.
Research suggests that undergoing varicocelectomy during adolescence may significantly enhance testicular growth in affected individuals. After treating varicocele in adolescents, an increase in testicular volume has been reported. Specifically, in adolescents with preoperative left testicular hypotrophy, a 69% growth in size was observed within 28 months following the varicocelectomy (21, 22).
In relation to the right testis volume, sperm count, motility, and morphology were positively correlated, while viability negatively correlated, again with no statistical significance. Finally, a positive correlation existed between sperm count, motility, viability, and morphology with the left testis volume. However, only between sperm motility and left testicular volume was the difference statistically significant. Kurtz et al. found that men with left-sided varicocele and a total testicular volume of less than 30 cc had four times the risk of having a low motile sperm count (23).
In our study, we observed that a decrease in left testicular volume among men with recurrent varicocele was linked to a lower motile sperm count. The results indicated that the count and motility of sperm were positively correlated with the diameter of the right and the left testicular veins. In a study conducted by Mehraban et al. in 2012 (24), the aim was to determine whether venous diameter could predict improvements in semen parameters following varicocelectomy. This study involved 85 patients who underwent bilateral laparoscopic varicocelectomy. The findings indicated a relationship between the diameter of the testicular vein and improvements in sperm motility, which aligns with the results of our study.
The relationship between sperm parameters following surgery and backflow is outlined as follows: Sperm count and motility exhibited significant differences with right backflow when combined with the Valsalva maneuver. Additionally, sperm count showed a significant difference with left backflow when using the Valsalva maneuver. Furthermore, there were significant differences in sperm count, motility, and morphology with left backflow when the Valsalva maneuver was not applied.
This study was limited by its single-center design and small sample size (n = 38), which may limit the generalizability of the findings. The short follow-up period (three months) limits conclusions about long-term fertility outcomes such as spontaneous conception or sustained improvements. Although semen analysis was performed manually and continuously by one operator, the lack of blinding may have introduced bias. Furthermore, factors such as time to conception, partner fertility status, and lifestyle influences were not controlled or fully assessed.
5.1. Conclusions
Our findings suggest that laparoscopic varicocelectomy significantly improves sperm count and motility, although changes in viability and morphology were not statistically significant. Anatomical factors such as testicular volume, venous diameter, and retrograde flow showed significant correlations with semen parameters, highlighting their predictive value. Given the variability in patient outcomes, further large-scale, long-term studies are needed to clarify the role of varicocelectomy in improving male fertility and achieving successful pregnancies.