1. Context
Efficient drug delivery with nanocarriers for effective and safe therapy has been developed during the last decades. Nanomaterial-based drug delivery systems (DDS) can deliver drugs to the target diseased cells in a controlled release manner. Nanomaterials (NMs) and nanoparticles (NPs) can be defined as the material with structures on the nanoscale dimension (between 1 and 100 nm) (1). As known, NMs and NPs exhibit tunable and unique physicochemical, mechanical, electrical, and biological characteristics. These special properties are due to the decreased size and increased surface area of them. As a result of the large surface area to volume ratio, NMs show quantum effects and special properties (2). Currently, nanomedicine has received attention by utilizing NMs and nanostructures as DDS (3). Drug delivery systems can deliver drugs to target tissues in a controlled release manner. Drugs can be chemically conjugated or physically encapsulated in these nanocarriers (4-6). The small size and high surface area of nanomaterials allow them to enter cells and interact with biomolecules easily. Improving absorption, bioavailability, and stability can be achieved by using nanotechnology in drug delivery, and therefore overcome the defects of common DDS.
Nanostructured delivery carriers can protect encapsulated drugs from in vivo degradation. On the other hand, the first-pass effect can be prevented by using DDS, especially in the case of water-insoluble drugs (7). The most widely used nano-based drug delivery carriers are micelles (8, 9), liposomes (10, 11), carbon nanotubes (12), solid lipid nanoparticles (SLN) (13, 14), dendrimers (15), mesoporous silica NPs (16), gold NPs (17, 18), quantum dots (QD) (19), and superparamagnetic iron-oxide nanoparticles (SPIONs) (20, 21). Polymeric nanomicelles are widely used as drug delivery systems. Some notable advantages of these carriers are biocompatibility, biodegradability, ease of preparation, and good loading and delivery efficacy (22-24). Amphiphilic polymers can be easily self-assembled into nanomicelles. Diblock copolymers composed of hydrophilic and hydrophobic blocks with variable lengths can encapsulate hydrophobic drugs in the core of the nanomicelle and/or attach hydrophilic drugs on the surface in the aqueous medium (25). Some common polymers used in the composition of nanomicelles are poly(ethylene glycol) (PEG) (26), N-(2-hydroxypropyl) methacrylamide (HPMA) (27), poly(l-lactic acid) (PLA) (28), poly(lactic-co-glycolic acid) (PLGA) (29), polycaprolactone (PCL) (30), and chitosan (31, 32). Most polymeric nanomicelles investigated for drug delivery applications have been used successfully in cancer. Examples of these nanomicelles are Genexol PM (33), NK911 (34), NK105 (35), NK012 (36), and NC6004 (37).
Many anticancer drugs are hydrophobic compounds, and nanomicelles with amphiphilic copolymers can solubilize them without using harmful organic solvents. On the other hand, targeting ligands on the surface of micelles target a specific receptor on cancerous cells and increase anticancer efficacy of the drug by accumulation in the site of action, on top of cell uptake enhancement. Nanoliposomes (also known as lipid-based nanovesicles) are versatile DDS made of bilayer lipids with an aqueous reservoir. This composition allows the delivery of hydrophilic and hydrophobic drugs. Nanoliposomes are good candidates for various applications in nanomedicine and nanobiotechnology because of their safety, stability, biocompatibility, and biodegradability. In comparison with polymeric nanomicelles, nanoliposomes have an improved drug release profile. Some nanoliposome formulations in drug delivery studies are DOXIL/Caelyx (38), Myocet (39), Depocyt (40), Daunoxome (41), CPX-1 (42), and CPX-571 (43).
One of the most important aspects of nanoscale DDS is the ability of targeting, which makes a smart DDS that only affects the diseased tissue. Nanoscale targeted drug delivery systems increase the concentration of the drug in the site of action, improve its efficacy, and reduce adverse side effects of the encapsulated drug. These advantages have led to targeted nanoscale DDS being highly regarded in research and therapeutics (44-46). The knowledge about diseases at the molecular level will help us in better targeting DDS development. Some modifications on the surface of DDS can cause a better accumulation of DDS near the diseased cells and improved uptake into the cells. These modifiers or ligands have widely been used in drug delivery investigations as targeting agents. This strategy is known as active targeting. In this approach, the imposed ligands on the surface of DDS bind with high affinity to specific components on the surface of diseased cells. Some targeting moieties investigated include antibodies, folate, lectins, peptides, aptamers, transferrin, lactobionic acid, oligosaccharides, and albumin (47).
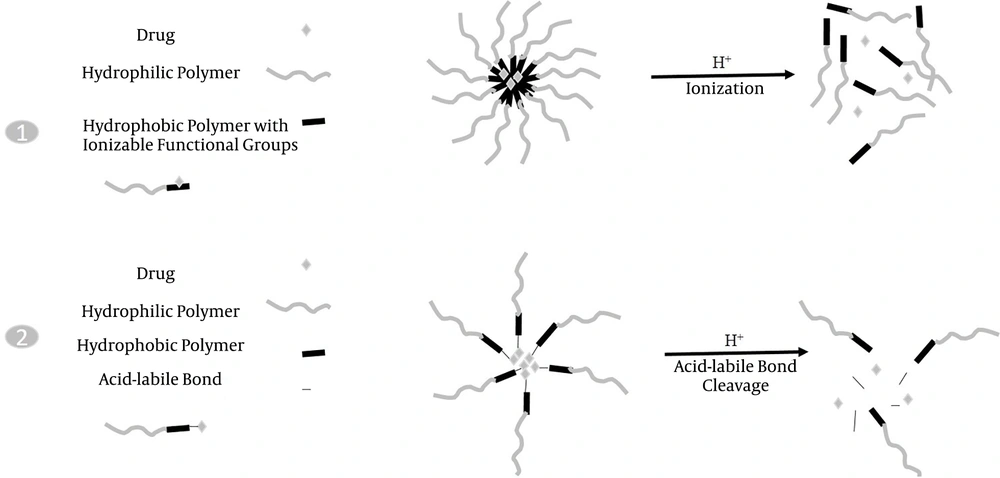
Nanostructured DDS can also provide controlled drug release by applying some modifications in the composition. Uncontrolled drug release may lead to the release of the encapsulated drug in the wrong site and consequently reduce the appropriate amount of the drug in the target tissue and increase its adverse side effects. In the case of nanoliposomal carriers, hydrogel embedded inside the liposome could successfully control the release of the drug. Another approach is the pH-sensitive release with polymeric nanomicelles in cancer therapy. These nanocarriers release the drug specifically in an acidic pH of the tumor environment. Polymers with ionizable functional groups are unstable in response to pH variations and thus release the encapsulated or conjugated drugs by structure destruction. Another manner is an acid-labile linker that can be used between copolymers and the drug to achieve controlled release in tumoral acidic pH. These two pH-sensitive release manners are shown in Figure 1. Some acid-labile bonds investigated in controlled release studies are hydrazone, oxime, imine, orthoester, and vinyl ether bonds. These two approaches have been extensively studied (48, 49), and the results have shown that in the latter approach, the drug release was lower than in the former approach because the polymeric structure of DDS was not destructed. Advances in nanoscale DDS are presented in this review.
2. Evidence Acquisition
The current review presents a summary of some advances in the development and application of nano-delivery systems for improving the efficacy of conventional drugs and reducing their adverse effects through the production of smart delivery carriers with targeting moieties and controlled release strategies used in therapy. The literature searches were conducted in ScienceDirect, Scopus, Google Scholar, and PubMed databases for relevant research articles up to 2020. The following keywords were used to identify the relevant articles: Drug delivery systems, nanocarriers, nanomaterials, nanomedicine, targeting delivery, and controlled release.
3. Results
The results listed in Table 1 were extracted from selected original reports.
| Nanocarrier + Drug | Indication (s) | Outcomes |
|---|---|---|
| Liposomal daunorubicin (DaunoXome®) (50) | Kaposi’s sarcoma | Enhanced delivery to the tumor site; Decreased side effects and systemic toxicity. |
| Liposomal amphotericin B lipid complex (Abelcet®) (51) | Fungal infections | Decreased toxicity |
| Micellar estradiol (Estrasorb™) (52) | Menopausal therapy | Improved efficacy by controlled delivery of the therapeutic agent. |
| Micellar DOX-Hyd-PLA-PEG-FOL (53) | Ovarian cancer | Targeted and controlled release of the drug, improved anticancer efficacy, decreased systemic toxicity. |
| Albumin-bound paclitaxel Nanoparticles (Abraxane®) (54) | Breast cancer; NSCLC; Pancreatic cancer | Enhanced solubility; Increased delivery to the tumor. |
| Morphine sulfate nanocrystals (55) | Psychostimulant | Improved drug loading and bioavailability; Slow release. |
| Paliperidone palmitate nanocrystals (56) | Schizophrenia; Schizoaffective disorder | Extended-release of drugs with low solubility. |
| Dantrolene sodium nanocrystals (57) | Malignant hypothermia | Administration of higher doses of the drug. |
| Ferumoxytol SPION with polyglucose sorbitol carboxymethylether (58) | Chronic kidney failure with iron deficiency | Decreased number of doses, extended-release |
| Micellar P123-PAE/Cur (59) | Cancer therapy | Longer circulation, pH-responsive controlled release, increased accumulation at the tumor site. |
| Micellar DTX-LEV-Hyd-PLA-PEG-FOL/DTX (60) | Ovarian cancer | Targeted and controlled release of the drug, improved anticancer efficacy, decreased systemic toxicity. |
| mPEG-TK-PLGA/ atorvastatin (61) | Acute kidney injury | Great ability to ROS-responsive drug release, target mitochondria, superior antioxidant, and anti-apoptotic activity. |
| C-dots/ transferrin+ epirubicin, Temozolomide (62) | Glioblastoma brain tumors | Targeted delivery, improved anticancer efficacy, synergistic effects by two-drug combination in the nanocarrier. |
| SLN + gelling system/MOX (63) | Psychostimulant | Improved encapsulation efficiency, controlled release of the drug, prolonged action in a sustained manner. |
Abbreviations: DOX, doxorubicin; Hyd, Hydrazine; PLA, poly(lactic acid); PEG, polyethyleneglycol; FOL, folate; P123, (EO20PO70EO20) amphiphilic block copolymer; PAE, poly(β-amino ester); Cur, curcumin; DTX, docetaxel; LEV, levulinic acid; TK, thymidine kinase gene; PLGA, polylactic-co- glycolic acid; SLN, solid lipid nanoparticles; MOX, moxifloxacin; NSCLC, non-small cell lung cancer.
4. Conclusions
The current review highlights advances in drug delivery through NM-based drug delivery systems. The common types of advanced nanocarriers include liposome formulations, polymeric carriers, micellar formulations, nanocrystals, protein nanoparticles, and many others for efficient transport and release of drugs at the disease site. A growing number of research studies in this area showed that nano-delivery carriers play an important role in the improvement of some types of therapy and can effectively overcome the problems of solubility, bioavailability, stability, targeting, and controlled release, as well as decrease side effects of drugs. Active targeting and stimuli-responsive controlled release of drugs are very useful strategies for smart drug delivery by nanocarriers. However, the complexity of in vivo environments can influence the efficacy of nanoscale delivery carriers for treatment purposes. However, with the deep and precise study of the molecular mechanism of diseases and more advances in nanotechnology, the DDS and their applications in nanomedicine can be improved. Nanomedicine is among one of the most attractive science branches, and in recent years, a great deal of research has been done on drug delivery systems based on nanotechnology. Despite many advances in the synthesis and application of drug carriers, there are still weaknesses. More pharmaceutical studies are needed in the case of size, composition, surface functional groups, drug loading, targeting agents, and controlled release factors in future nanocarriers. Another approach is to consider the potentially hazardous effects of nanocarriers on humans and the environment. In other words, risk and benefit must be considered simultaneously. Although many studies have been done in this area, additional research is needed. The future of nanomedicine with targeted delivery and controlled release of drugs is a promising therapy.