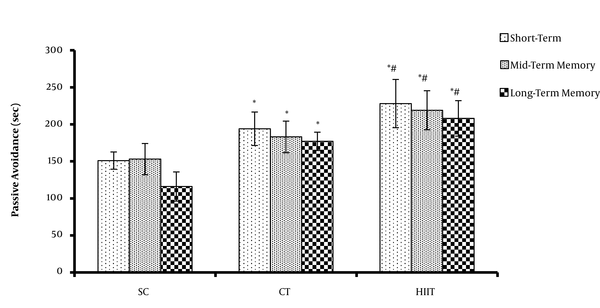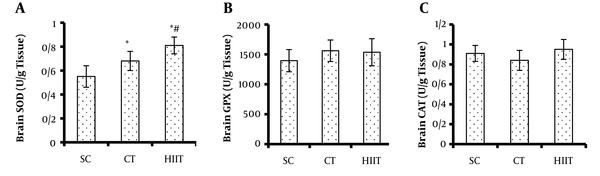1. Background
It has been considered that exercise contributes to brain health by promoting synaptic plasticity, antioxidant properties, and survival of neurons (1-3). Improved brain health and memory function are the products of enhanced antioxidant systems and subsequently lowered oxidative stress effects in exercise training (4).
Superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX) act as the main antioxidant enzymes that scavenge reactive oxygen species (5, 6). The literature shows contradicting results concerning the response of antioxidant enzymes to exercise training. According to the evidence, 12 weeks of high-intensity interval running resulted in more GPX activity, compared to moderate-intensity continuous running, in adult patients with type 2 diabetes mellitus; however, no dramatic changes were observed in SOD activity (5).
Moreover, it has been demonstrated that continuous training (CT) at moderate intensity and interval training at high intensity similarly increase SOD concentration in the cardiac tissue of infarcted rats; nevertheless, the increase in GPX following interval training is significantly superior to that of the continuous one (7). A reduction in oxidative stress responses to short-term high-intensity interval training (HIIT) was attributed to improved CAT status in healthy subjects (8). In another study, an improved SOD activity with no significant change in GPX was shown following resistance training at moderate intensity in untrained men (9).
At present, exercise training is recognized as an efficient and low-cost tool to counteract and treat several neurological diseases (10, 11). In contrast to many pharmacological approaches for the treatment of memory loss, exercise training has no serious side effects. In reality, prescribing the best approach needs more understanding of the effects of different exercise training regimes on memory.
2. Objectives
Therefore, the current study aimed to firstly compare the effects of high-intensity interval and continuous protocols on memory function and secondly demonstrate their associations with antioxidant enzyme activity in the rat brain.
3. Methods
3.1. Animals
This experimental study was reviewed and approved by the Ethics Committee of Sport Sciences Research Institute of Iran (code: IR.SSRC.REC.1399.101). For this study, 18 male albino Wistar rats (5 months old) were provided from the Animal Laboratory at Zahedan University of Medical Sciences, Zahedan, Iran, and randomly assigned to three equal (n = 6) groups of sedentary control (SC), CT, and HIIT.
3.2. Continuous and Interval Exercise Training Protocols
Both CT and HIIT regimens were followed in the Animal Laboratory of Zahedan University of Medical Sciences for 6 consecutive days per week for 6 weeks, according to Table 1 (11).
| Training | Days | Protocol | Overload |
|---|---|---|---|
| Continuous training | Even | 27 m/min (80% VO2max) | 2 min each day reaching 60 min in the following 4th week |
| High-intensity interval training | Even | 54 m/min for 30 sec (100% VO2max) and active rest for 60 sec at 16 m/min; active rest was realized between intervals for 60 sec at a speed of 16 m/min | 3 repetitions and 4.5 min each day while increasing to 20 repetitions and 30 min in the 4th week |
| Odd | 40 m/min for 3 min (95% VO2max), while the active recovery phase was 60 sec at 16 m/min with 2 repetitions and 8 min. | 6 repetitions and 24 min in the 4th week |
Continuous Training and High-Intensity Interval Training Protocols
3.3. Retention of Passive Avoidance Task
The rats were subjected to a one-trial step-through paradigm in grid floor and wooden-wall shuttle box immediately after the last session of CT and HIIT regimens at the end of the protocol. The shuttle box consists of a light and dark compartment with a dimension of 80 × 20 × 20 cm (12). The assessment of short-, mid-, and long-term memory was carried out by registering latency in entering the dark compartment following 6, 24, and 48 h after a three-second electric shock to the foot (AC, 60V, 1 mA current at 50 Hz), respectively (12, 13).
3.4. Biochemical Assays
The animals were sacrificed by decapitation (ketamine, 60 - 80 mg/kg; xylazine, 8 mg/kg; IP) 48 h after the last exercise session (10). The freshly removed brain was washed by normal saline and rapidly submerged in liquid nitrogen for 1 min before it was stored at -80°C. A total of 40 - 55 mg of powdered brain tissue by liquid nitrogen was dissolved and homogenized in 1 mL of 1X phosphate-buffered saline containing 1% Protease Inhibitor Cocktail (#GB-326-1, ProBlockTM-50, Gold Biotechnology CO, USA) to inhibit protease activity (11). The mixture was thoroughly vortexed (StuartMixers Vortex, SA8TM, England) and centrifuged (Eppendorf Centrifuge, Mini SpinR, Germany) at 5000 × g at 2°C - 8°C for 5 min, and the supernatant of the brain was collected. The SOD, GPX, and CAT activities and malondialdehyde (MDA) level in the supernatant were measured using a commercial kit (BiocoreDiagnostik Ulm GmbH, Veltlinerweg 29, Germany). The values were expressed in the gram of tissue weight.
3.5. Statistical Analysis
The SPSS software (version 23.0, SPSS Inc, Chicago, USA) was used for analysis. After screening with the Shapiro-Wilk and Levene’s tests, one-way analysis of variance, Bonferroni post hoc, and Pearson’s correlation coefficient test were used.
4. Results
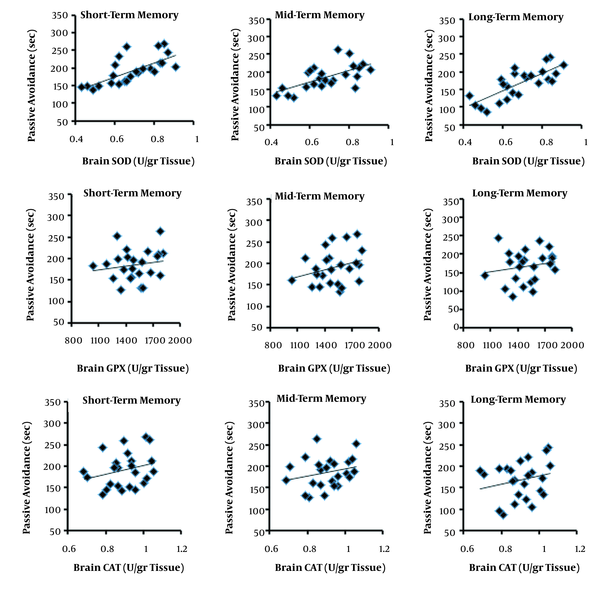
Figure 1 illustrates the results for short-, mid-, and long-term memory. Both HIIT and CT regimens increased short-, mid-, and long-term memory, and the alterations were greater following HIIT than CT. In addition, Figure 2 depicts brain SOD, GPX, and CAT activities. Both HIIT and CT regimens significantly increased SOD activity, with a higher elevation following HIIT than CT. Nevertheless, no difference was shown for GPX and CAT between the groups. Figure 3 shows the correlation of brain antioxidant enzyme activity with short-, mid-, and long-term memory. Brain SOD activity positively correlated with short-, mid-, and long-term memory. However, neither CT nor HIIT had a significant effect on brain GPX and CAT activities. In addition, a comparison of brain MAD levels among SC, CT, and HIIT groups is illustrated in Figure 4. indicating no difference between the groups.
Comparison of brain superoxide dismutase (SOD) (A), glutathione peroxidase (GPX) (B), and catalase (CAT) (C) activities among sedentary control (SC), continuous training (CT), and high-intensity interval training (HIIT) groups; asterisk (*) indicating a significant difference between groups (P < 0.05).
5. Discussion
Both HIIT and CT improved short-, mid-, and long-term memory and brain SOD in rats; nonetheless, the HIIT regimen had a greater impact than CT. However, brain GPX, CAT, and MDA levels did not significantly change in the HIIT and CT groups. Moreover, a significant positive correlation was observed between brain SOD activity, as an antioxidant marker, and short-, mid-, and long-term memory, as a cognitive function.
Several studies have shown that exercise has the potential to enhance memory function in healthy and anxious patients (3, 14). The findings of the present study are in line with the results of other studies suggesting an improvement in both short- and long-term memory following exercise training (3, 14). In addition, it has been reported that forced treadmill exercise prevents cognitive deficit in the Morris spatial water maze task following chronic cerebral hypoperfusion in rats (15). In two human studies, it was demonstrated that running on the treadmill at moderate (14) and high intensity (3) with a short recovery period enhanced short- and long-term episodic memory.
Moreover, the findings of the current study demonstrated an improvement in memory function following both interval and continuous running exercise at high intensity. Less escape of learned vocabularies from memory following cycle ergometer at high intensity than low intensity has been attributed to the greater release of neurotrophic factor from the brain (16). It has been shown that exercise-induced neurotrophic factor in various cortical areas (17) mediates memory progress by increasing cerebral oxygenation and neurogenesis (1). In addition, it has been demonstrated that the positive effects of long-term supervised exercise on verbal learning and memory are mediated by the enhancement of cardiovascular fitness in middle-aged adults (16).
In a study, the improvement of memory function has been attributed to reversing hippocampus volume loss following long-term aerobic exercise (18). Significant increases in both gray and white matter regions of the prefrontal cortex in exercise training are associated with cognition enhancement and healthy functioning of the central nervous system in sedentary community-dwelling volunteers (19). Finally, van Praag et al. (20) have reported enhancement in learning, retention, and hippocampus neurogenesis in aged rodents by voluntary wheel running.
It has been assumed that exercise-induced cognitive improvements are mediated by enhancement in lowering lipid peroxidation (21) and brain antioxidant system (22). The results of the present study demonstrated that both intensive interval and continuous running on the treadmill increased brain SOD activity. Consistent with the results of the present study, it has been shown that swimming training (8 weeks; 5 days/week; 30 min/day) attenuates brain MDA levels by increasing brain SOD activity in Wistar rats (23). Furthermore, Souza et al. have reported that 60 min swimming per day for 6 weeks attenuates reactive oxygen species by raising SOD activity in the cerebral cortex of rats (22).
In a study conducted by Shirvani et al. (24), the levels of SOD significantly increased after a session of low-, moderate-, and high-intensity training while preventing the increase in the MDA level of the cerebral cortex. However, the benefits of high-intensity training were higher than others. The aforementioned study investigated the impact of an acute exercise session; in addition, memory function was not assessed (24).
Nevertheless, the findings of the present study are inconsistent with other studies in which the insignificant change in SOD activity in the hippocampus, striatum, and cerebral cortex of stroke rat models is attributed to low-to-moderate-intensity exercise (15). The SOD converts the superoxide anion into a molecule of hydrogen peroxide and oxygen, thereby minimizing the formation of hazardous free radicals and lipid peroxidation (25). Brain-derived neurotrophic factor (BDNF) brings about an increase in the expression of SOD in angiogenic cells (26).
In addition, it has been shown that the continuous infusion of BDNF inhibits the acute down-regulation of SOD expression in old rats suffering from spinal cord injury (27). Through an increase in SOD activity, BDNF shields against free-radical-mediated excitotoxicity injury, which sequentially decreases MDA levels (28) and improves memory function (23). Therefore, the findings of the current study propose that the changes in brain SOD may correlate with enhancing brain BDNF following interval training than CT.
The results of the present study showed no significant change in brain CAT and GPX activities following both intensive interval training and CT. Inconsistently, a study demonstrated a more significant increase in GPX activity in intensive interval training than continuous moderate-intensity training in a post-myocardial infarction rat model (7). Although GPX and CAT activities were reduced in the hippocampus of streptozotocin-induced rats with diabetes mellitus, the activities of these antioxidant enzymes have been shown to significantly increase after an eight-week running protocol on a treadmill at low to moderate intensity (29). Overall, the discrepancy in results may, at least in part, be due to the differences in subjects and tissue type.
Marcus et al. (30) have reported that SOD, GPX, and CAT antioxidant enzyme activities significantly reduce in frontal, temporal, and cerebellar cortex regions of patients with Alzheimer’s disease. Moreover, a correlation has been shown between non-enzyme antioxidant improvements and memory progression in old and very old subjects (31). In this regard, the present study revealed a significant positive correlation between brain SOD enzyme antioxidant and short-, mid-, and long-term memory following intensive exercise training.
In another study, it has been revealed that MDA high levels are associated with impairment in visual-spatial and auditory-verbal working memory as well as short-term and delayed declarative memory (21). Additionally, it is believed that improvement in cognitive performance following forced treadmill exercise is mediated by a reduction in hippocampus MDA levels in stroke rat models (15). However, it seems that the results of the current study were not affected by lipid peroxidation since brain MDA levels did not change following intensive running.
5.1. Conclusions
Intensive interval training can bring about more enhancements in rat’s short, mid-, and long-term memory through enhancement in brain SOD level. This finding suggests that dividing training sessions into various bouts of exercise with maximum effort may result in substantial memory gains.