1. Background
Parkinson’s disease (PD) is one of the most common neurodegenerative diseases in the world. The cause of this disease is the dopaminergic neuronal degeneration in the substantia nigra pars compacta (SNpc). It could be due to environmental and genetic factors (1). Different mechanisms have been proposed to play a critical role in this process. Mitochondrial impairment (2), oxidative stress factors, free radicals (3), and aggregation of alpha-synuclein have been implicated in PD pathogenesis (4). The production of reactive oxygen species (ROS) is an important factor for neuronal death in the PD. The studies have revealed the inhibition of the complex I of the electron transport chain in the mitochondria and the increase of the oxidative stress enzymes (ROS) are the earliest events, followed by modifications of alpha-synuclein and proteasomal dysfunction, leading finally to neurodegeneration (1). The brain is susceptible to generating ROS and oxidative stress factors, due to its high levels of metabolism, along with the high content of strong oxidizing molecules, such as neuromelanin and dopamine, which their metabolisms produce ROS (5).
The use of dopamine precursor levodopa (L-DOPA) is considered a standard treatment for Parkinson’s disease. Very few percentages of levodopa are transmitted from the blood-brain barrier. This dopamine precursor will be able to activate dopaminergic receptors. It, therefore, can compensate for the reduction of dopamine present in the brain (6). Levodopa is quickly transformed to dopamine by the enzyme of decarboxylase at the outside of the brain. High doses of levodopa result in high dopamine production outside the brain, which will be responsible for causing side effects of excessive drug use (7, 8).
The advantage of using levodopa in combination with other medicines (i.e., levodopa and benserazide) is to reduce the side effects of increasing dopamine in the environment, such as nausea, vomiting, and cardiac arrhythmias. The use of combined levodopa therapy is suitable for those patients who cannot tolerate the daily dose of levodopa . Benserazide reduces the levodopa decarboxylation in the environment. Therefore, most of them enter the brain and prevent the increase of dopamine in the peripheral tissues (6). Clinical studies have shown that the levodopa to benserazide ratio of 4 to 1 is best for reducing peripheral dopamine and increasing dopamine in the brain. Levodopa is widely distributed in most tissues and it crosses the blood-brain barrier (9). Benserazide do not have the ability to cross the blood-brain barrier. Abnormal movements such as choreiform or dystonic movements are related to levodopa therapy. Muscle twitching is one of the first symptoms of increasing drug dosage (10). However, with the advancement of the disease and the loss of more dopaminergic neurons, the brain will be disturbed to compensate for and maintain the physiological level of dopamine after levodopa intake (6, 11). This leads to motor fluctuations, dyskinesia, and emotional deterioration (12). The incidence of such problems in the motor system is related to several variables, such as age-related illness and drug intake time. Studies have shown that the use of high dose levodopa leads to more severe dyskinesia in patients, which is a sign of more lesions in the brain (9).
2. Methods
2.1. Experimental Protocol
The rats were randomly divided into four groups (n = 7 each). The control group received only saline. The second group received 2 mg MPTP toxin per nostril as a PD model. The third group was treated by levodopa (10 mg/kg) intraperitoneally one week after PD for 14 days. The fourth group received combined levodopa (10 mg/kg) and benserazide (2.5 mg/kg) intraperitoneally one week after PD for 14 days. All rats were decapitated four weeks after the last behavioral test, and their brains were prepared for Immunohistochemistry, including staining of tyrosine hydroxylase (TH), histochemistry, and TUNNEL investigations.
2.2. Animals
Twenty eight male albino Wistar rats (weighing 200 - 250 g) at the beginning of the experiment were provided by the experimental center of Semnan Medical Sciences University, Semnan, Iran. All rats were housed in cages with free access to food and water. The temperature of the storage room was maintained at 20ºC - 23ºC in simulated daylight conditions (12-h dark and 12-h light). All stages of testing were carried out in accordance with the Ethics Committee of Semnan Medical Sciences University.
2.3. Intranasal Administration of MPTP
The neurotoxin 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) HCl (Sigma) was used by intranasal (i.n.) administration according to the procedure described by Prediger et al. (13). Briefly, the rats were lightly anesthetized with ketamine and xylazine hydrochloride (75 mg/kg - 15 mg/kg) (Sigma-Aldrich). A 10-mm piece of a plastic tube (PE 50) was inserted into the nostrils. The tubing was connected to a peristaltic pump to maintain a constant flow rate of 10 mL/min. The MPTP HCl was initially dissolved in ethanol (10% w/v) and then in NaCl) 0.9 % (at a concentration of 2 mg/mL, followed by infusing by a peristaltic pump for 4 min (2 mg/nostril). The control solution consisted of saline. The animals were given a one-minute break to resume their normal respiratory function and then this procedure was repeated with infusions administered through the contralateral nostrils.
2.4. Pole Test
Rats, as well as other rodents, use their forepaws for the greatest “activities of daily living,” and the basal ganglia determine many aspects of forepaw activity but have a low impact on hind limb movement (14). Studies reveal that the pole or grid test focuses on forepaw activity, so it can discover each disability in the dopaminergic system (15). One day prior to testing, the animals were allowed to adapt to the environment and test. The testing machine consisted of a 60-cm high wood with a diameter of 1 cm, a cork ball with a diameter of 1.5 cm at the top, and a base that was positioned on a flat surface. The animals were placed head upward directly below the cork ball. This test requires motor coordination to turn downward and to climb to the ground. The time the animal took to reach the floor was determined at locomotors activity time, LAT. The pole test was performed before and after treatment.
The tests were repeated three times and their average values were calculated for statistical analysis.
2.5. Immunohistochemical Study
In order to investigate the neuronal dopaminergic population within the SNpc and striatum, the immunohistochemical study was done. The animals were deeply sedated with ketamine (100 mg/kg) and xylazine (15 mg/kg) and intracardially perfused with saline and 4% of the fixative solution of par formaldehyde (PFA) in 0.1 M phosphate buffer (pH: 7.4). The brains were removed and fixed in 2.5% PFA. Coronal sections of 6 µm were cut. First, tissue sections were blocked in 10% methanol and H2O2 for 8 min in darkness. Then, they were washed several times with Tris (pH: 7.4), incubated in citrate buffer (pH: 7.6) at 98ºC for 10 - 15 min, and washed with Tris (pH: 7.4) three times every time three minutes. They were then blocked in 10% normal goat serum, 1% BSA, and 0.3% triton X-100, in PBS for two hours at 25ºC. The sections were incubated with the primary antibody (ab 6211) dissolved in 0.3% TBS with 1% BSA (1:500) and reacted overnight at 4ºC. Next, they were washed three times every time 5 min in TBS. FITC-conjugated secondary antibody incubation (1:200 ab 214879) was performed for 2 h at room temperature (25ºC). Following several times washing in PBS, the sections were finally DAPI stained, washed in Phosphate buffered saline, dehydrated, and cover-slipped. After identifying the desired area of the SNpc and immunopositive cells, they were counted only when their nuclei were optically visible (16).
2.6. Nissl Staining
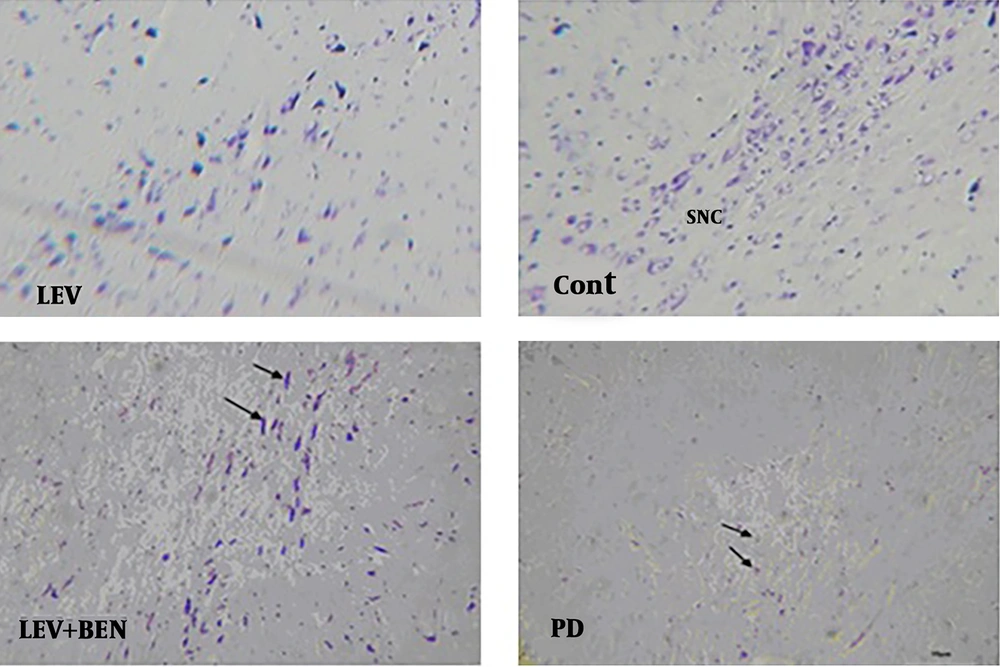
To evaluate histological changes, seven days after treatment, cresyl fast violet or Nissl staining was carried out on the 10-μm paraffin sections. The sections were immersed in 70% ethanol for at least two hours and then washed three times with distilled water. Then, the sections were incubated in cresyl violet solution (Beyotime Ins. Biotechnology) for three minutes and then dehydrated in 100% ethanol for one minute.
2.7. TUNNEL Staining
The DNA fracture of apoptotic cells was identified by TUNNEL staining. The procedure was according to the manufacturer’s instruction (Roche, Basel, Switzerland). Briefly, following three times washing in PBS, the sections were fixed for one hour with 4% par formaldehyde. Then, they were treated with 3% H2O2 in 70% methanol for 15 minutes and washed again with PBS three times. After that, the sections were treated with 0.1% Triton X- 100 solutions in 0.1% sodium citrate for three minutes on ice. The slides were washed with PBS. The Tunnel reagent solution (50 μL) was added to each sample and subsequently, the samples were incubated for one hour at 37ºC in a humidified atmosphere in darkness. Finally, they were counterstained with 4; 6-diamidino-2-phenylindole (DAPI) in PBS (Santa Cruz Biotechnology) for 15 sec. the stained sections were evaluated under a fluorescence microscopy (Olympus).
2.8. Statistical Analysis
All data were expressed as means ± SEM. The data were analyzed by one-way ANOVA followed by Tukey’s post hoc test for neuron counts. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Pole Test
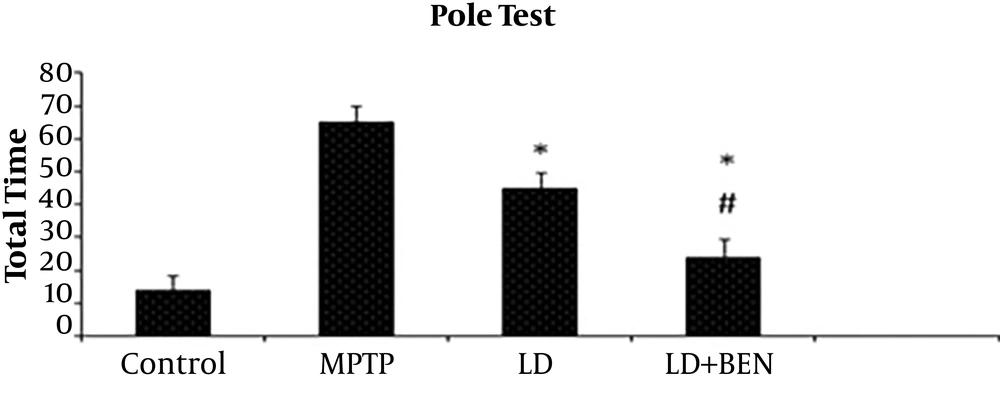
Behavioral results showed the total time of descending (TTD) decreased in third and fourth groups in comparison with the MPTP group (P < 0.05) (Figure 1). The TTD was 69.3 ± 15 sec in the MPTP group and 12.2 ± 6 sec in the control group. The results showed TTD in the MPTP group significantly increased in comparison with the control group (P < 0.05). In the third group that received only levodopa , the mean TTD was not significantly different from that of the MPTP group (46.9 ± 10 vs. 69.3 ± 15). In the fourth group that received levodopa plus benserazide, the TTD was significantly different from that of the PD group (23.4 ± 5 vs. 69.3 ± 12) (P < 0.05). A significant difference was identified between the third and fourth groups, as well (46.9 ± 10 and 23.4 ± 5, respectively) (P < 0.01) (Figure 1).
Pole test performance in the experimental groups: the total time was significantly affected by all treated groups compared to the MPTP group (P ≤ 0.05). In the levodopa plus benserazide, it was significantly different from that of the levodopa group (P ≤ 0.05). All values are means ± SEM. * Compared to the MPTP (PD) group (P ≤ 0.001). # Compared to the levodopa group P (P ≤ 0.001).
3.2. Immunohistochemistry
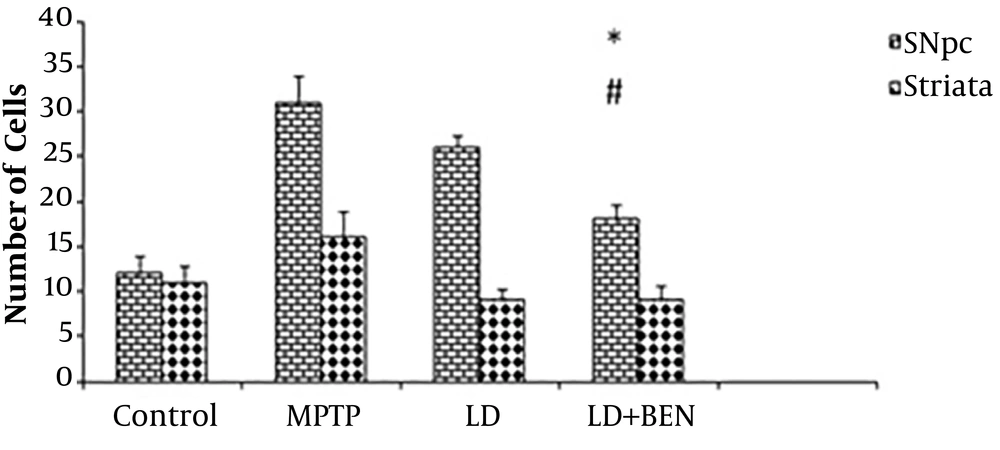
The MPTP treatment results showed a significant reduction in the number of TH positive cells in the SNpc and striatum, compared to the control group. However, treatment with levodopa alone did not prevent from MPTP-induced loss of TH-positive neurons. This is while the results in the combination therapy group were different. IHC staining revealed a significant reduction in TH- positive cells in the SNpc and striatum of the MPTP treated group compared to the control group (15 ± 1.2 and 11 ± 0.4 vs. 31 ± 1.5 and 17 ± 0.3; P < 0.001), as illustrated in Figures 2 and 3. The results showed that the use of medication (levodopa plus benserazide) could support the dopaminergic neurons. Although the number of neurons was higher in the combined treatment group than in the MPTP group, the difference was significant compared to the levodopa group. The number of TH-positive neurons in the SNpc of the levodopa plus benserazide group was 22 ± 1.2 and in that of the Levodopa group was 16 ± 0.6. The results showed that there was a significant difference in the number of TH-positive neurons between the combined treatment group and the levodopa alone treatment group (P < 0.05). The number of TH-positive neurons in the striatum of the levodopa plus benserazide group was 15 ± 1 and in that of the levodopa group was 13 ± 0.6. There were no significant differences between the treatment groups in the striatum. The results showed the maximum TH-positive cells were in the group of levodopa plus benserazide. TH staining showed a significant preservation of TH-positive cells in the levodopa plus benserazide group, suggesting that an increase in dopamine in the brain following the use of benserazide could support the dopaminergic neurons of the SNpc.
Immunohistochemical analysis of tyrosine hydroxylase (TH) positive cells in the SNpc and striatum of the rats’ brains. There is a significant difference in the average number of TH positive neurons between the SNpc of the combined treatment group and that of the MPTP group (P ≤ 0.05). There is no significant difference in the striatum of the treatment groups and that of the MPTP group. All values are means ± SEM. * Compared to the MPTP group (P ≤ 0.001). # Compared to the levodopa group (P ≤ 0.001).
3.3. TUNNEL Staining
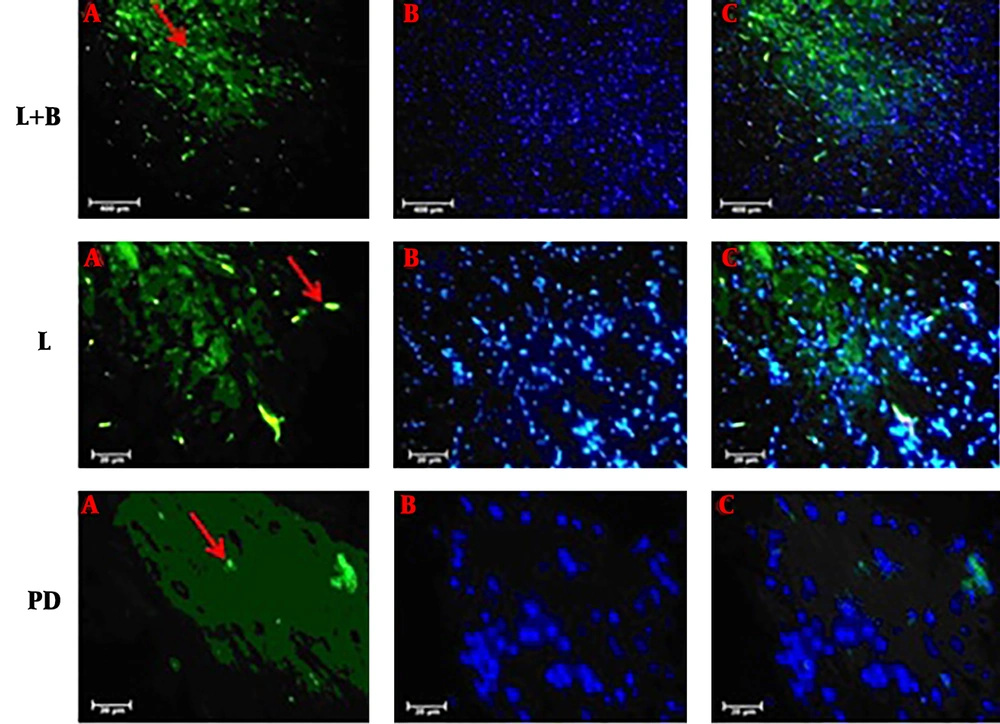
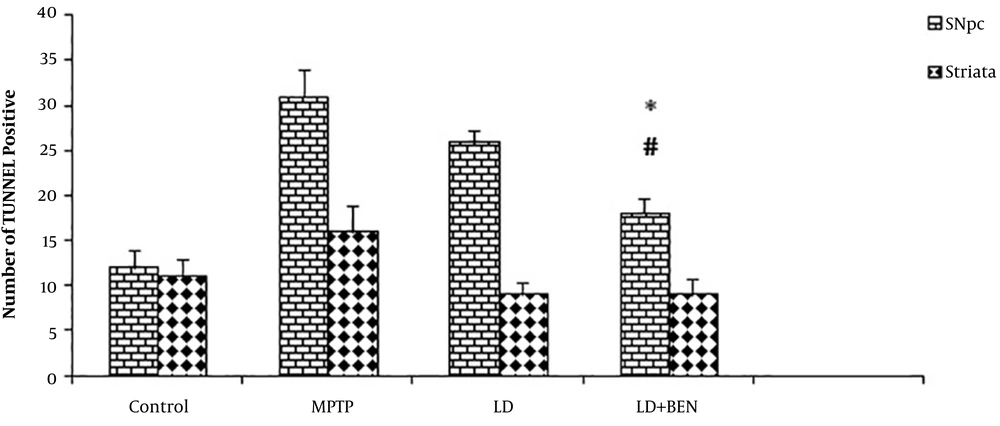
Previous studies have shown that the TUNNEL staining method is useful for identifying apoptotic neurons (17). We counted the TUNNEL positive cells in the SNpc and striatum areas (Figures 4 and 5). TUNNEL positive cells were detected in the SNpc and striatum of all groups. However, the number of TUNNEL positive cells was much higher in the MPTP group than in the other groups. The average number of TUNNEL-positive cells in the SNpc of the control group was 12 ± 0.9 and in MPTP was 31 ± 1.6. The TUNNEL staining method showed a significant induction of positive cells in the MPTP treatment. The results showed that the number of TUNNEL positive cells in the combined treatment group significantly reduced in comparison with the Parkinson’s group. The number of TUNNEL-positive cells in the levodopa group was 26 ± 0.8 and in the levodopa plus benserazide group was 18 ± 1.1. The results showed that there was a significant reduction in the number of TUNNEL-positive cells in the levodopa plus benserazide group in comparison with the MPTP and Levodopa treatment groups (P < 0.05). In the striatum, the results revealed that TUNNEL-positive cells significantly decreased in all treatment groups in comparison with the MPTP group. The number of TUNNEL-positive cells was 9 ± 0.2 in the Levodopa group, 9 ± 0.1 in the levodopa plus benserazide group, 16 ± 0.1 in the MPTP group, and 11 ± 0.2 in the control group. There were no significant differences between the treatment groups and the control group.
TUNNEL positive cells in the SNpc and striatum of the rats’ brains. There is a significant difference in the average number of TUNNEL positive cells between the SNpc of the combined treatment group and that of the MPTP group (P ≤ 0.05). There is no significant difference between the striatum of the treatment groups and that of the MPTP group. All values are means ± SEM. * Compared to the MPTP group (P ≤ 0.001). # Compared to the levodopa group (P ≤ 0.001).
3.4. Nissl Staining
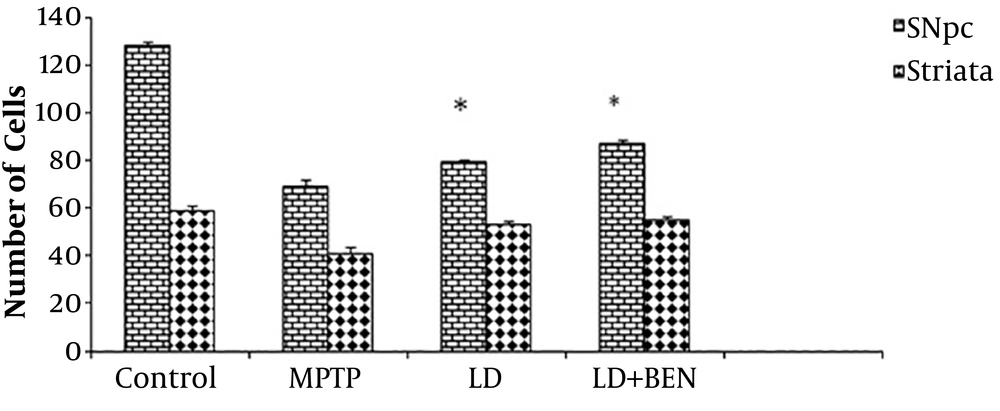
Nissl staining revealed a significant reduction in the neurons of the SNpc and striatum of the MPTP-treated group compared to the control group (69 ± 1.2 and 41 ± 0.4 vs. 128 ± 1.5 and 59 ± 0.3; P < 0.001), as illustrated in Figures 6 and 7. The number of intact neurons was higher in the combined treatment group (levodopa plus benserazide) than in the PD group, but the difference was not significant between the treatment groups (79 ± 1.4 and 87 ± 1.3). The number of neurons in the SNpc of the levodopa plus benserazide group was 87 ± 1.2. The results of the striatum are almost the same as those of the SNpc. The results of striatum showed that there was no significant difference in the number of neurons between the two treatment groups (P < 0.05). The results showed the maximum neurons were in the group of levodopa plus benserazide.
Nissl staining in the SNpc and striatum of the rats’ brains. There is a significant difference in the average of colored cells in the SNpc between all treatment groups and the MPTP group (P ≤ 0.05). There is no significant difference between the striatum of the treatment groups and that of the MPTP group. All values are means ± SEM. * Compared to the MPTP group (P ≤ 0.001).
4. Discussion
Conventional treatments for PD are limited in effectiveness and only reduce the symptoms of the disease, but they do not eliminate the cause. L-dopa, which increases the level of dopamine in the brain, has been used for a long time, and is currently considered a golden treatment for PD (18).
At present, the most commonly used drug for symptom control is levodopa that is used to reduce symptoms at all stages of the disease. However, the use of this drug increases the risk of non-permanent movements (dyskinesia) (6). In addition, its effectiveness reduces after four to five years, and even disappears; therefore, the need for new drug combinations is essential. The substantia nigra is vulnerable to oxidative damage. The main feature of PD is the loss of dopaminergic neurons in the substantia nigra (19). The first major finding of this study is that MPTP severely reduces dopaminergic neurons of the SNpc (8, 20). This study revealed that behavioral activity restored when levodopa plus benserazide was used. Since benserazide inhibits the peripheral decarboxylation of levodopa , it decreases its conversion to dopamine in peripheral tissues, causing more levodopa to enter the central nervous system to become dopamine. This may be the main cause of behavioral improvement (21). An increase in oxidative stress induction in the brain of Parkinson’s patients has been reported elsewhere (19, 22).
Dysfunction of mitochondrial complex I results in a decrease in ATP production, increase in oxidative stress, remodelation of mitochondrial morphology, demolition of calcium buffering, damage to mitochondrial DNA, and change in mitochondrial fission and fusion, finally leading to cell death. Alterations in mitochondrial complex I are the main source for ROS generation in PD (23). The mitochondrial respiratory chain stopped by the inhibition of mitochondrial complex I leads to incomplete oxygen reduction, thereby generating reactive species containing noxious O2-, which is further converted to NO3- and finally, to OH via the Fenton reaction. Thus, mitochondria with dysfunctional activity are the primary source of intracellular ROS contributing to oxidative stress-mediated neurodegeneration in PD models (15, 19). Our finding revealed that the number of apoptotic cells was lower in the combined treatment group than in the other treatment group. In addition, immunohistochemical staining showed the number of TH-positive cells increased in the SNpc of the combined treatment group. In addition, the results showed there was a positive correlation between behavioral improvements and the number of TH-positive cells. Therefore, it is possible that an increase in levodopa in the brain decreases brain oxidative stress enzymes and this leads to behavioral improvement .Increasing the amount of Levodopa in the brain reduces the number of apoptotic cells. Therefore, there is a direct correlation between the level of brain levodopa and cell death. This can be due to decreased levodopa and its metabolites in the serum.
The results showed that the use of medication (levodopa plus benserazide) could support the dopaminergic neurons, suggesting that an increase in dopamine in the brain following the use of benserazide could support the dopaminergic neurons of the SNpc. We emphasize that an increase in the level of dopamine in the brain and a reduction in serum dopamine metabolites can support dopaminergic neurons in the short-term.