1. Background
H2O2 plays a prominent role in different areas including clinical diagnostics, food industry, pharmaceutical, and environmental protection (1). An excessive accumulation of H2O2 in the human body can lead to some diseases such as DNA fragmentation and tissue damage (2). Consequently, the accurate measuring of H2O2 in biological samples is very important. To date, various analytical methods have been developed for determination of H2O2, including chromatography (3), spectrophotometry (4), and chemiluminescence (5). However, above mentioned techniques are high costs, complex, and time-consuming.
Electrochemical H2O2 sensing has attracted growing attention due to the intrinsic advantages such as simple operation, cost effective, rapid response, and suitability for real-time H2O2 analysis (6). In view of this, the design and fabrication of novel electrochemical sensors for H2O2 determination has attracted considerable interest in recent years (7, 8).
2. Objectives
In this study, novel V2O5/VO2 nanostructures were successfully prepared with the hydrothermal method. The V2O5/VO2 nanostructures were utilized for modification of a simple and cheap carbon paste electrode (CPE). The main experimental factors, calibration range, and detection limit of the V2O5/VO2-CPE was also explored in detail. Moreover, the proposed sensor was applied for quantification of H2O2 in human serum samples.
3. Methods
3.1. Materials and Apparatus
All materials including thiourea, vanadium chloride (VCl3), ethanol, ethylene glycol, paraffin oil, graphite powder, and H2O2 were obtained from Merck (Darmstadt, Germany). XRD pattern was recorded via a PANalytical Empyrean X-ray diffractometer (Almelo, Netherlands). SEM image and EDX analysis were performed on a MIRA3 LM TESCAN microscope (Brno, Czech Republic). FT-IR spectrum was measured on a Bruker Equinox 55 spectrometer (Karlsruhe, Germany). All electrochemical data were recorded on a potentiostat galvanostat impedance meter (OrigaState100, OrigaLys, Rillieux-la-Pape, France). A standard electrochemical cell including the modified CPE (working electrode), the platinum wire (counter electrode), and the Ag/AgCl (3 M KCl) electrode (reference electrode) was used for electrochemical tests.
3.2. Preparation of V2O5/VO2 Nanostructures
The V2O5/VO2 nanostructures were synthesized via a hydrothermal approach. Typically, 7.8 g of VCl3 was dissolved in a mixed solution of ethanol (50 mL) and ethylene glycol (25 mL) under vigorous stirring. Afterward, 7.6 g of thiourea was added to the above mixture vigorous stirring. The mixture was transferred into a 100 mL Teflon-lined stainless-steel autoclave and then heated at 160ºC for 12 h. The black precipitates were collected via centrifuge separation, followed by repeated washing with ethanol, and then heated at 350ºC for 3 h under air condition.
3.3. Electrode Fabrication
The modified CPE (V2O5/VO2-CPE) was fabricated by mixing of graphite powder (65% w/w), V2O5/VO2 (10% w/w), and paraffin oil (25% w/w). Then, a portion of the obtained paste was packed into a glass tube (3 mm in diameter) in contact with a copper wire for electrical connection.
4. Results
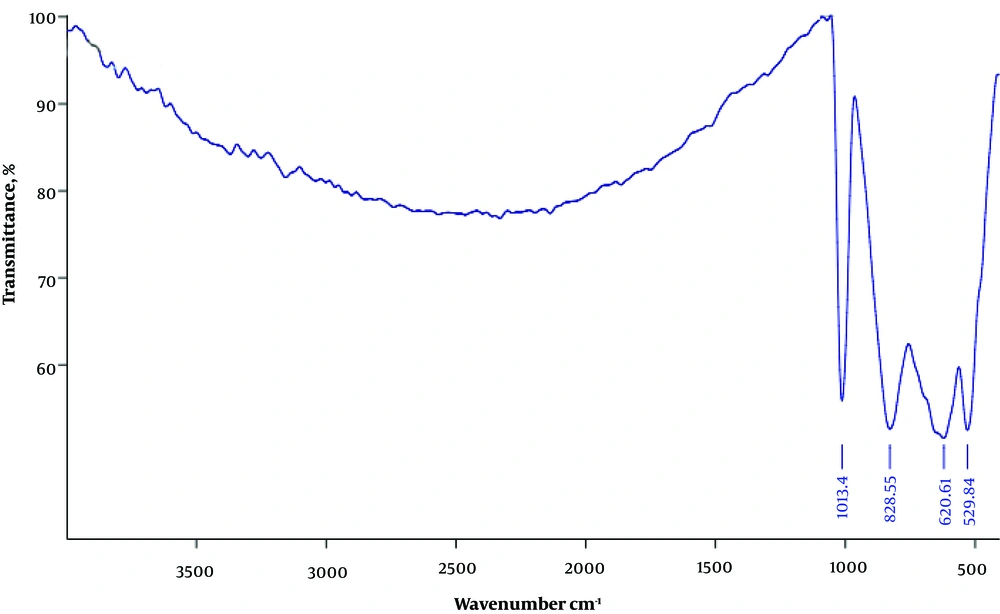
The FT-IR spectrum of the V2O5/VO2 nanostructures is presented in Figure 1. Absorption peak appearing at 1013 cm-1 is attributed to the stretching vibrations of V=O groups (9). The peaks at 529 and 828 cm-1 are related to the symmetric and asymmetric stretching of the V−O−V groups, respectively (10). The peak at 620 cm-1 can also be assigned to the stretching of the V−O groups (11).
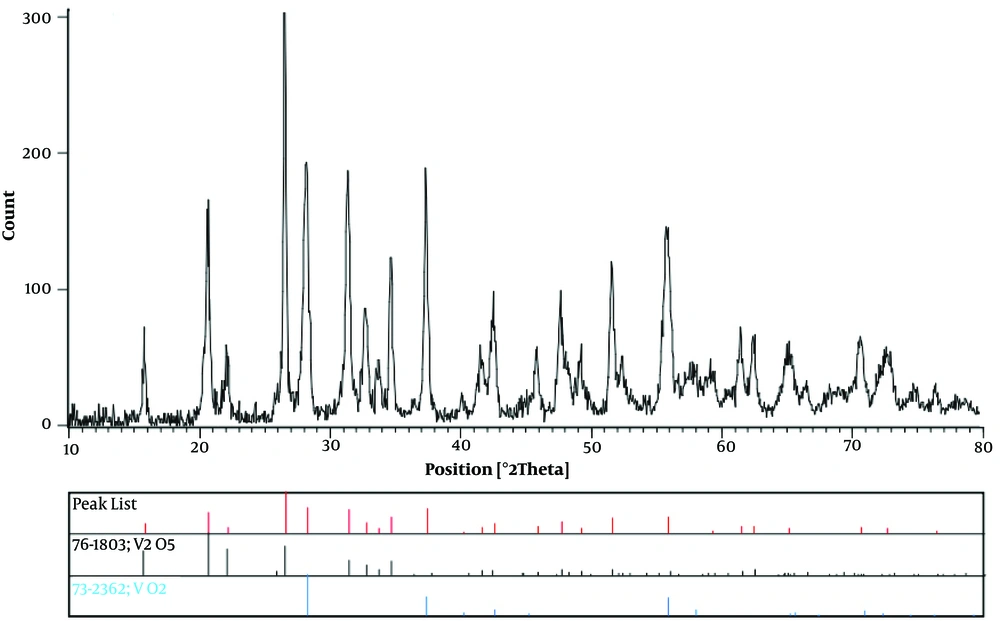
XRD pattern of the V2O5/VO2 nanostructures is shown in Figure 2. The observed characteristic reflections were matched well with the standard XRD data of V2O5 (vanadium pentoxide; JCPDS No 76-1803) and VO2 (vanadium dioxide; JCPDS No 73-2362) (12).
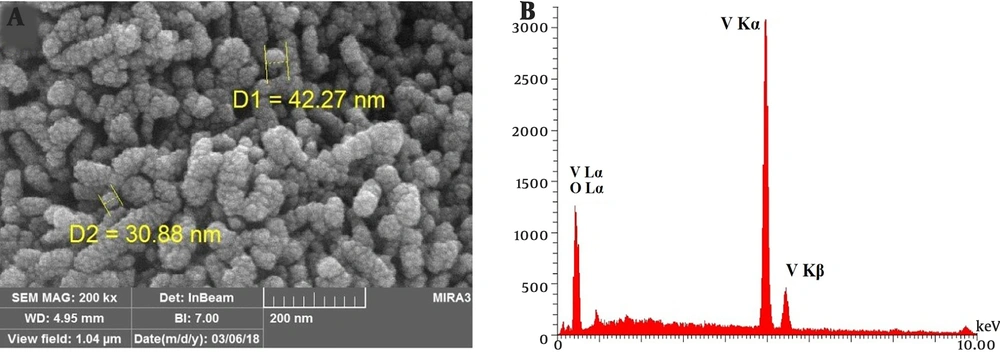
The SEM photograph of the V2O5/VO2 nanostructures is shown in Figure 3A. As can be seen, the prepared V2O5/VO2 product has a nano-sized structure. Moreover, the EDX spectrum of the V2O5/VO2 nanostructures (Figure 3B) shows the existence of vanadium and oxygen elements in the prepared material. According to these results, the V2O5/VO2 nanostructures have been successfully prepared by hydrothermal method.
5. Discussion
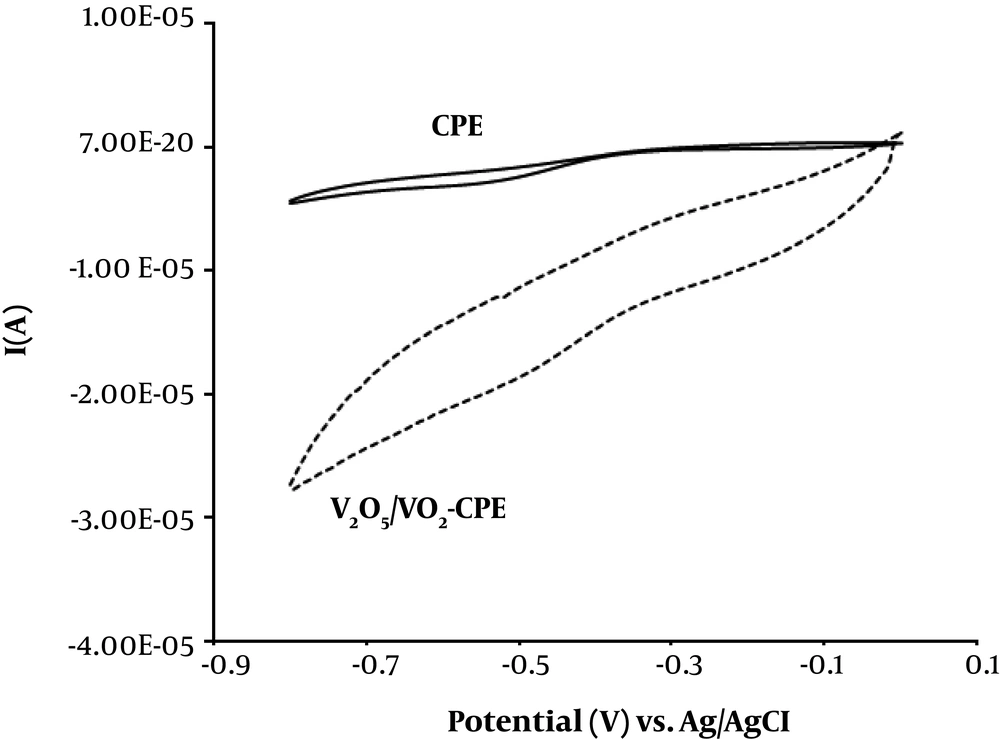
The ability of the V2O5/VO2 nanostructures for electrochemical reduction of H2O2 was investigated. Figure 4 displays the cyclic voltammograms of unmodified CPE and V2O5/VO2-CPE in phosphate buffer (0.1 M, pH = 7) containing 100 μM of H2O2. As can be seen, the V2O5/VO2-CPE shows a notable reduction peak current, indicating that the reduction of H2O2 was improved compared to unmodified CPE.
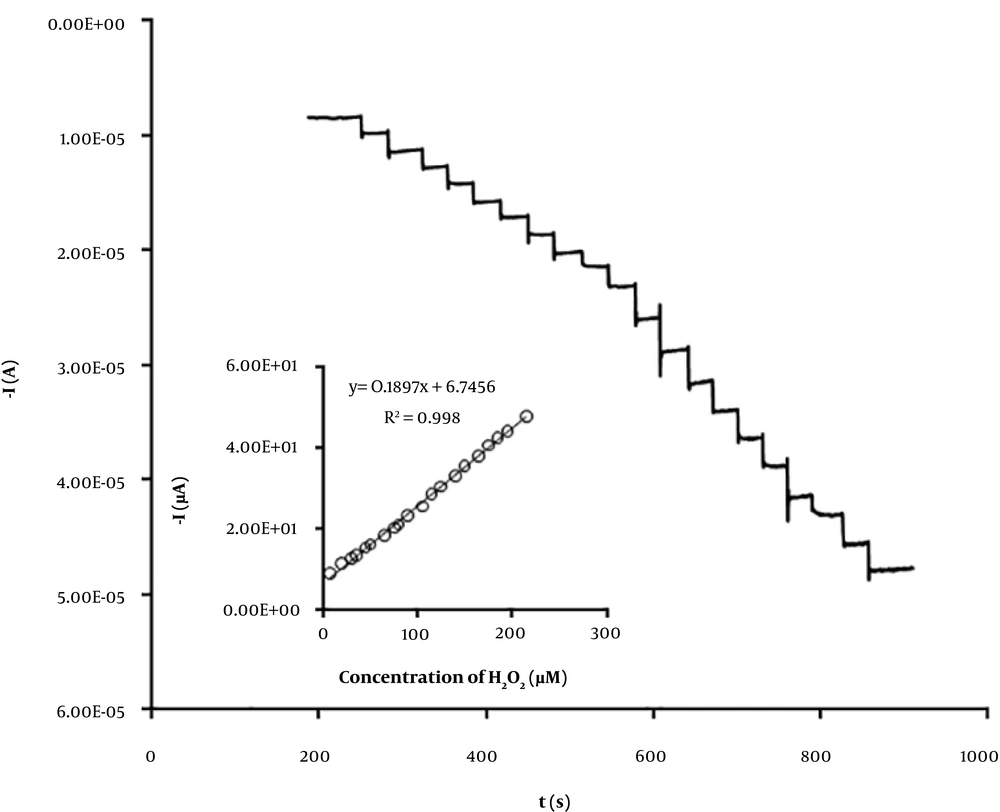
To improve the sensitivity of the V2O5/VO2-CPE toward H2O2 detection, amperometry method was used at applied potential of -450 mV. The amperometric responses of the V2O5/VO2-CPE to the successive addition of H2O2 were studied as shown in Figure 5. The amperometric signal currents vary linearly with H2O2 concentration over the range of 8 to 215 μM with a detection limit (3σ) of 5 μM. The detection limit of the prepared sensor is lower than some reported H2O2 sensors (13-17), as listed in Table 1. The effect of some interfering species including glucose, uric acid, dopamine, and ascorbic acid was also investigated. The experimental results (Table 2) show that the prepared V2O5/VO2-CPE can measure H2O2 with recoveries more than 95% in the presence of such interfering molecules.
| Electrode Modifier* | Linear Range, µM | Detection Limit, µM | Ref. |
|---|---|---|---|
| Cuprous oxide-reduced graphene oxide (Cu2O-rGO) nanocomposites | 30 - 12800 | 21.7 | (13) |
| Poly (p-aminobenzene sulfonic acid) (PABS) | 50 - 550 | 10 | (14) |
| Magnetite (Fe3O4) nanoparticles | 25 - 5000 | 7.4 | (15) |
| Hematite (α-Fe2O3) nanoparticles | 50 - 3145 | 22 | (16) |
| Iodide | 10 - 60000 | 10 | (17) |
| V2O5/VO2 nanostructures | 8 - 215 | 5 | This work |
Comparison of Some Different Electrochemical Sensors for Determination of H2O2
| Co-existing Molecule | Recovery, % |
|---|---|
| Glucose | 96.7 |
| Uric acid | 98.5 |
| Dopamine | 98.3 |
| Ascorbic acid | 96.9 |
The Effect of Interfering Species
The suggested V2O5/VO2-CPE was validated for the H2O2 quantification in biological samples. Human serum samples were collected from a local hospital in Kerman. Standard addition protocol was used in the recovery experiments and the obtained results are listed in Table 3.
| Sample | Added, µM | Found, µM | RSD | Recovery, % |
|---|---|---|---|---|
| Male serum | ||||
| 15 | 14.8 | 4.2 | 98.6 | |
| 30 | 29.5 | 3.6 | 98.3 | |
| 45 | 46.3 | 5.0 | 102.8 | |
| Female serum | ||||
| 15 | 15.6 | 4.5 | 104.0 | |
| 30 | 28.9 | 5.2 | 96.3 | |
| 45 | 44.2 | 3.9 | 98.2 |
Determination of H2O2 in Human Serum Samples (n = 3).
5.1. Conclusions
In summary, a novel electrochemical sensor for determination of H2O2 was presented. The fabricated H2O2 sensor showed a wide linear range and low detection limit. The applicability of the V2O5/VO2-CPE for quantification of H2O2 in biological samples was successfully investigated. The good simplicity and anti-interference performance of the present method indicates that the V2O5/VO2-CPE has great potential working as an efficient H2O2 sensor for clinical applications.