1. Background
The sedentary lifestyle in adults is associated with overweight and obesity, and recognized as the fifth leading cause of death in the world. It increases the risk of diseases such as hypertension, metabolic syndrome, and cardiovascular disease (1). In inactive people, obesity and weight gain are strongly associated with changes in the lipid profile and insulin resistance as the risk factors of cardiovascular disease (2). Studies show that the main causes of atherosclerosis and heart disease include increasing C-reactive protein (CRP) as an inflammatory marker (3), very low-density lipoprotein (VLDL), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and triglyceride (TG) along with decreasing a potent marker called high-density lipoprotein (HDL-C) (4). Also, it seems that entering a college, because of the sensitivity of this important period of life, can cause a major change in the mental and physical lifestyle of girls, which can be linked to weight gain, obesity, and increased risk of cardiovascular disease (5). Studies have shown that physical activity is effective in lowering blood lipids, reducing inflammatory factors, and improving the immune system (1, 4, 6, 7). Based on studies, regular, long-term moderate and low-intensity endurance training has beneficial effects on reducing inflammation, decreasing insulin resistance, and improving aerobic metabolism (8).
In recent years, researchers in the field of sports science have become more interested in research into the cardiovascular effects of high-intensity interval training (HIIT) (6). However, contradictory results have been reported on the intensity and the type of training. For example, continuous and interval training had a significant effect on fibrinogen and CRP in healthy obese women (9). Moreover, HIIT training had a significant effect on the reduction of decreased LDL, TC, increasing HDL, and decreasing hs-CRP in sedentary healthy overweight/obese youth (10). In addition, HIIT and continuous training decreased LDL, TC, increasing HDL, and decreasing hs-CRP serum levels, but the effects were not significant on TG and VLDL (11). On the other hand, HIIT and continuous training did not have a significant effect on reducing CRP serum levels in obese children (12) and HIIT increased intracellular adhesion molecules (13).
2. Objectives
Given that the motivation for physical activity has diminished due to the occupation and modernization of societies and the growing prevalence of obesity can increase the risk of cardiovascular disease, especially in young women, finding a suitable method that can be the most appropriate way to prevent these diseases in the shortest possible time seems indispensable. Accordingly, this study aimed to investigate the C-reactive protein and lipid-lowering effect of HIIT training in physically active (PhA) and physically inactive (PhI) women.
3. Methods
This was a quasi-experimental study. Forty volunteer female college students of Islamic Azad University of Behbahan (mean age 22 years and 10 months, mean height 160 cm, and mean weight 57.9 kg) were selected based on availability. They were briefed on the purpose of research after completing a consent form. It is noteworthy that before the grouping of subjects, they completed the physical health questionnaire and the International Physical Activity Questionnaire (IPAQ), reliability of which had been measured by researchers in Iran with a correlation coefficient of 0.86. The inclusion criteria for all subjects were no history of cardiovascular disease, no smoking, not having at least six months of regular exercise, and the ability to perform the training. Based on the IPAQ, the subjects were categorized into three levels (sedentary, adequate mobility, and high mobility). Students with physical activity levels of less than 600 METs-min/week were classified as low physical activity group (inactive) and between 600 and 3000 and higher as appropriate physical activity group (active group). It is noteworthy that this questionnaire was designed according to a study by BashiriMoosavi et al. (14).
The subjects included 20 PhI students (control and HIIT groups) with no athletic experience at least in the last two years and 20 PhA students (control and HIIT groups). Afterward, before the start of training in the pretest and 48 hours after the last training session in the posttest, blood samples (6 cc) were gathered from the left brachial vein of each subject at 8:00 to 9:00 a.m. During six weeks, three sessions per week, the HIIT groups performed HIITs CRP (Iranian Biotechnic kit) measured by Hitachi 911 device with enzymatic photometry and lipid profiles measured by Pars Azmoon kit.
During six weeks, the HIIT groups performed the training for three sessions per week over a distance of 20 m (indicated by cones) (Figure 1). Indeed, each subject first ran from cone 1 to cone 2 (path A) and then backed to cone number 3 (path B) and eventually to cone number 1 (path C). Each subject did this process for 30 seconds and then rested for 30 seconds. For training overload, 30-second intervals were increased in the first and second weeks (four intervals), third and fourth weeks (six intervals), fifth and sixth weeks (six intervals). Subjects were instructed to warm up and cool down for 5 minutes before and after training. The training protocol of the present study was designed based on a 40 m shuttle run test with maximum speed running. Training intensity was above 90% of the maximum heart rate (HRmax) at all stages. It should be noted that the “220-age” formula was used to calculate the HRmax (15). The Kolmogorov-Smirnov test was used for assessing the normal distribution of data. The one-way analysis of variance (ANOVA) with Tukey’s post hoc test was used for statistical analysis of data (P ≤ 0.05).
4. Results
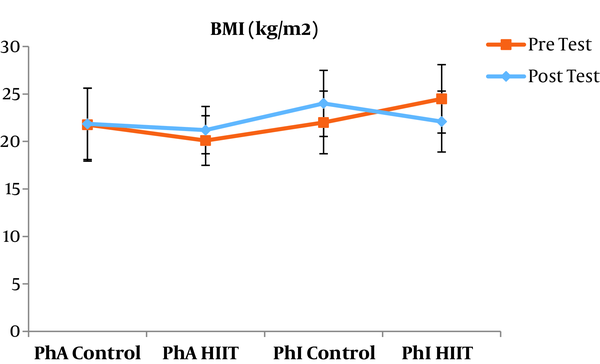
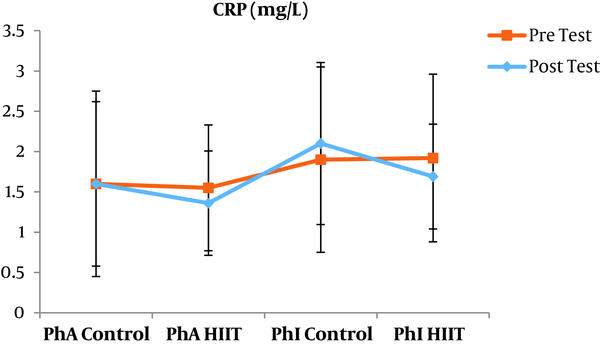
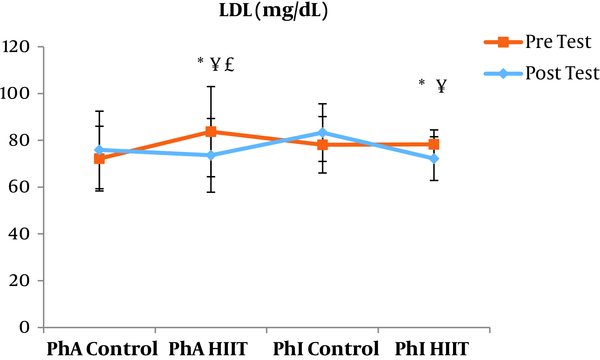
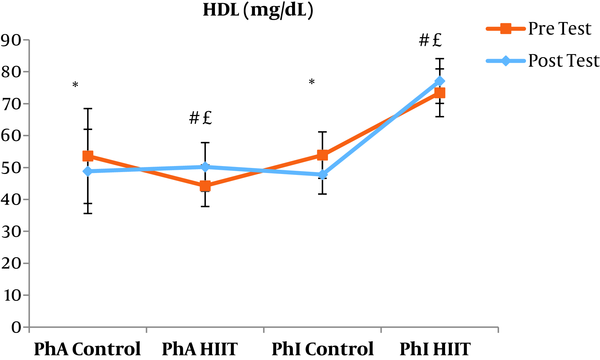
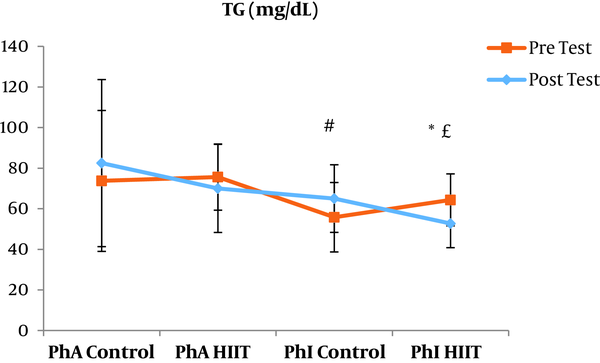
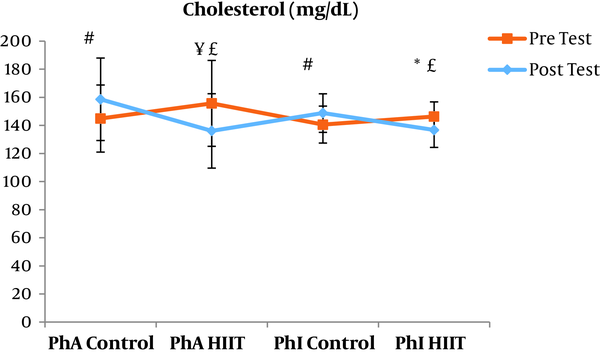
The results of the paired sample t-test showed that body mass index (BMI), CRP, LDL, and TG were not significantly different between the post-intervention and pre-intervention in the PhA control group (P ≥ 0.05). However, VLDL and cholesterol were significantly higher in the post-intervention than in the pre-intervention in the PhA control group (P ≤ 0.05) and HDL (P = 0.002) was significantly lower in the post-intervention than in the pre-intervention (Figures 2 - 8). In the PhA HIIT group, BMI, TG, cholesterol, and CRP did not significantly change from the pre-intervention to post-intervention (P ≥ 0.05); however, LDL and VLDL were significantly lower in the post-intervention than in the pre-intervention (P ≤ 0.05) and HDL was significantly higher in the post-intervention than in the pre-intervention (P = 0.002). In addition, BMI, LDL, VLDL, and CRP were not significantly different between the post-intervention and pre-intervention in the PhI control group (P ≥ 0.05), but HDL (P = 0.01) was significantly lower in the post-intervention than in the pre-intervention. In the PhI control group, TG and cholesterol were significantly higher in the post-intervention than in the pre-intervention (P ≤ 0.05). In the PhI HIIT group, BMI and CRP were not different between the post-intervention and pre-intervention (P ≥ 0.05); however, LDL, VLDL, TG, and cholesterol were significantly lower in the post-intervention than in the pre-intervention (P ≤ 0.05). Also, HDL in the PhI HIIT group was significantly higher in the post-intervention than in the pre-intervention (P = 0.03).
The results of the one-way ANOVA test indicated no significant difference in BMI (Figure 2) and CRP (Figure 3) between the research groups (P ≥ 0.05). However, there were significant differences in LDL, VLDL, TG, cholesterol, and HDL between the research groups (P ≤ 0.05). The results of Tukey’s post hoc test showed that LDL was significantly lower in the PhA HIIT (P = 0.002) and PhI HIIT groups (P = 0.03) than in the PhA control group. In the PhA HIIT (P = 0.001) and PhI HIIT (P = 0.01) groups, the LDL levels were significantly lower than that of the PhI control group (Figure 4).
Moreover, VLDL was significantly lower in the PhA HIIT (P = 0.02) and PhI HIIT (P = 0.02) groups than in the PhA control group. The VLDL levels were significantly lower in the PhI HIIT group than in the PhI control group (P = 0.02) (Figure 5). The HDL levels were significantly higher in the PhA HIIT (P = 0.001) and PhI HIIT (P = 0.001) groups than in the PhA control group. The HDL levels were significantly higher in the PhA HIIT (P = 0.001) and PhI HIIT (P = 0.001) groups than in the PhI control group (Figure 6). The TG level in the PhI HIIT group was significantly higher than in the PhA control group (P = 0.01) and lower than in the PhI control group (P = 0.009) (Figure 7). The cholesterol levels were significantly lower in the PhA HIIT group than in the PhA control group (P = 0.001) and PhI control group (P = 0.005) and they were significantly lower in the PhI HIIT group than in the PhA control group (P = 0.02) (Figure 8).
5. Discussion
The levels of LDL, VLDL, and cholesterol were significantly lower and the HDL levels were significantly higher in the PhA HIIT and PhI HIIT groups than in the PhA control group. Nevertheless, changes in CRP and TG levels were not significant in the PhA HIIT, PhI HIIT, and PhA control groups. Low physical activity has been known to increase the risk of various diseases such as atherosclerosis, diabetes, hypertension, and so on (15). Most studies showed the effect of long-term and aerobic exercise on weight loss and lipid profile improvement in obese and inactive individuals (4, 8, 16).
There are contradictory results regarding the effect of HIIT on the lipid profile. For example, HIIT significantly reduced the percentage of lipid, cholesterol, and LDL and increased HDL levels in a study (17). This study was consistent with the present study regarding the reduced LDL and cholesterol levels and inconsistent regarding the HDL variations. The similarity of the training period and the inactivity of both populations appear to be the reasons for the consistency of the results. Also, since BMI was not significantly different between different groups in the present study, some of the results of this study can be justified based on the study by Batacan et al. (18); probably because of the baseline levels of lipid profile in normal-weight individuals and even in some overweight people, HIIT with different intensities had no significant effect on HDL, LDL, VLDL, and TG changes. However, it was noted in the present study that interval training in obese and overweight individuals may have lipolyzed visceral adipose tissue and released lipid metabolites into the bloodstream (18).
In the present study, the LDL, VLDL, and TG levels were significantly lower and the HDL levels were significantly higher in the PhI HIIT group than in the PhI control group. Nevertheless, the differences in CRP levels were not significant between the PhI HIIT and PhI control groups. It has been reported that HIIT significantly lowered the levels of LDL cholesterol, fat percentage, BMI, and cardiovascular risk factors (19). Moreover, HIIT significantly decreased TG, LDL, and total cholesterol levels and increased HDL (20). Besides, HIIT decreased TG and increased insulin sensitivity, but had no significant effect on cholesterol, HDL, and LDL variations (21). In addition, one session of resistance training and continuous training had no significant effect on CRP in overweight men (22); however, Pilates and endurance training reduced CRP in obese and elderly individuals (23). Differences in the type and intensity of training appeared to be effective in reducing CRP and the differences in these two factors may be the reasons for the difference of this study with the present study.
In coronary artery disease (Atherosclerosis), the elevated levels of HDL can have protective roles and induce the reverse transport of cholesterol from peripheral vascular tissue to the liver (24). The HDL-C levels appear to play a major role in the process of reverse cholesterol transport systemic circulation. Because the reverse transport of cholesterol leads to the process of removing excess cholesterol from peripheral tissue, an increase in plasma HDL concentrations may be effective in lowering cholesterol levels (25).
One of the possible causes of elevated HDL is weight loss and decreased plasma triglycerides, which appear to result in insulin improvement. One possible cause of increased HDL is the increase in lipoprotein lipase (LPL) as a result of exercise. Lipoprotein lipase is effective in converting VLDL to HDL; thus, by increasing its activity, the HDL levels increase; increased HDL can be due to the increased production in the liver and changes in various enzymes such as lecithin cholesterol acyltransferase and decreased hepatic lipase activity following exercise (26). Exercise also appears to improve metabolism, increase insulin sensitivity, improve endothelial function, and result in weight loss. Given the limitations of the measurement of some cytokines such as interleukin-6 and its direct association with serum levels of CRP, it seems that the lack of measuring this factor would be a limitation to the present study. Therefore, it is proposed to consider this variable in future studies. Given that the measured markers to prevent cardiovascular disease in individuals were inactive, the lack of the measurement of cardiac waves was the limitations of this study. Therefore, it is suggested that future studies evaluate cardiac waves in addition to these variables.
5.1. Conclusions
Although the lipid profile and CRP increase in inactive people, it appears that HIIT has favorable effects on lipid profile in PhA and PhI women. Nevertheless, given the unchanged level of some markers, further studies are needed.