1. Context
Pregnancy is a complex physiological state characterized by profound immune changes that facilitate successful fetal development. A successful pregnancy depends on accurate and coordinated communication between the fetus and the mother. Immune cells and cytokine signaling pathways play a prominent role as mediators of this communication (1). During pregnancy, the maternal immune system undergoes changes to tolerate the fetus. Natural killer (NK) cells are known as the key immune cells of the uterus during pregnancy, primarily covering the entire endometrium. In normal pregnancy, these cells do not exhibit cytotoxic properties and play a significant role in implantation and placental regulation (2, 3). Macrophages (Mφ), one of the main subsets of leukocytes in the decidua region of the uterus, perform a unique function in creating the immunological aspects of the interaction between mother and fetus due to their phenotypic flexibility (4, 5). Regulatory T cells (Tregs), a vital component of the T lymphocyte family, play a crucial role in maintaining immunological tolerance and regulating immune responses in both healthy and pathological processes. Research indicates that Tregs prevent the development of the maternal immune response against the fetus (6). Pregnancy and delivery are regulated by cytokines, and a disruption in the balance of these hormones can lead to difficulties such as autoimmune illnesses or microbial infections. This disorder may also cause recovery from autoimmune disease during pregnancy with recurrence after delivery (7, 8). Preeclampsia, spontaneous abortion, and intrauterine growth restriction (IUGR) are common pregnancy complications often resulting from abnormal placentation and impaired placental function. In preeclampsia, impaired trophoblast invasion and inadequate remodeling of maternal spiral arteries lead to reduced placental blood flow (9, 10). The imbalance between antiangiogenic factors such as sFlt-1 and sEng contributes to endothelial dysfunction, resulting in the clinical manifestations of preeclampsia (11). In cases of spontaneous abortion, defective trophoblast invasion and reduced HLA-G expression may trigger maternal natural killer (NK) cell activation, leading to fetal rejection (12). For IUGR, placental insufficiency and diminished uteroplacental blood flow deprive the fetus of oxygen and essential nutrients (13). These disorders not only increase the risk of fetal mortality but are also associated with long-term metabolic and cardiovascular complications in childhood and adulthood (14). Therefore, it is important to expand knowledge to understand the complexities of common immune regulatory pathways in pregnancy to improve and develop new strategies for the treatment of immune-based infertility. Understanding the interactions between NK cells, Tregs, and cytokines is crucial to elucidate the underlying mechanisms in successful reproductive processes and address pregnancy-related complications. This study investigates the function of NK cells, the vital role of Mφs during healthy pregnancy, and the function of cytokines and Tregs in balancing maternal and fetal immunity during healthy pregnancy.
2. Natural Killer Cells in Pregnancy: Supporting Fetal Growth and Maternal Health
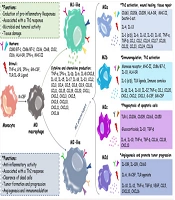
Natural killer (NK) cells constitute approximately 15% of all circulating lymphocytes in humans. These cells possess inherent cytotoxic qualities and are characterized by their large size and granule-containing nature. The NK cells secrete inflammatory mediators such as interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α), which contribute to cytotoxicity and the generation of inflammatory cytokines (15, 16). Phenotypically, NK cells are characterized by the expression of surface receptors cluster of differentiation (CD) 56 and CD16. Based on the concentration of CD56 antigen, NK cells can be divided into two groups: CD56dim and CD56bright. The CD56dim type exhibits high cytotoxicity in vitro, while the CD56bright type produces important immune cytokines such as IFN-γ (17). Typically, decidual natural killer (dNK) cells exhibit the CD56brightCD16- phenotype, whereas peripheral blood NK (pbNK) cells exhibit the CD56dimCD16+ phenotype (18). In humans, NK cells are exclusively found in the decidua region of the uterus; thus, dNK and uterine NK (uNK) cells are synonymous (19). The exact origin of uNK cells remains unknown. These cells may arise from the translocation of pbNK cells to the decidua and the presence of local hematopoietic precursor cells (HPC), as suggested by a study conducted on mice. The HPCs isolated from the decidua can develop into uNK cells in the presence of interleukin (IL) 15, which is the primary factor for uNK cell activation and maturation (19, 20). The population of blood leukocytes in the endometrium undergoes changes during menstruation, with the highest number observed in the secretory phase and the lowest in the proliferation phase. At the end of the secretory phase and the onset of pregnancy, the population of uNK cells increases rapidly, comprising about 70% of uterine leukocytes. This population reaches its peak in early pregnancy, but their numbers decrease as pregnancy progresses towards the trimester stage (21). The performance of NK cells is determined by the balance between signals received by activating and inhibitory receptors (22). The growth of the placenta and maintenance of the placental bed are crucial for fetal development throughout pregnancy, relying on fetal trophoblast invasion and the presence of immune cells such as uNK cells. It is evident that uNK cells are important for both trophoblast invasion and spiral artery regeneration, as they are frequently observed near regenerating spiral arteries and invading fetal trophoblast cells (23). During trophoblast invasion, trophoblasts separated from the embryo invade the decidua and uterine wall, leading to vascular changes in the uterine endometrium, known as decidualization. Meanwhile, uNK cells proliferate and settle at sites of trophoblast invasion. Extravillous trophoblasts (EVTs) derived from the fetus and maternal uNK cells regenerate maternal vessels and repair spiral arteries, allowing maternal blood to flow into the placental villi to supply nutrients and oxygen needed by the fetus (24, 25).
2.1. Types of Decidual Natural Killer Cells Based on RNA Sequencing
Decidual tissue-resident markers such as CD49a and CD9 can be expressed by dNK cells, which can be categorized into three groups based on RNA sequencing: dNK1, dNK2, and dNK3. The dNK1 group expresses Beta-1,4-N-Acetyl-Galactosaminyltransferase 1 (B4GALNT1), CD39, and cytochrome P450 Family 26 Subfamily A Member 1 (CYP26A1). CD39 is a regulatory ecto-ATPase that helps shift the environmental equilibrium from pro-inflammatory to anti-inflammatory. Additionally, this group shows increased expression of activating receptors KIR2DS1 and KIR2DS4, which bind to HLA-Cs in trophoblasts, as well as inhibitory killer cell immunoglobulin-like receptors KIR2DL1, KIR2DL2, and KIR2DL3, which also bind to HLA-Cs in trophoblasts. The dNK1 subgroup may interact with EVTs, as evidenced by the expression of leukocyte immunoglobulin-like receptor B1 (LILRB1), a receptor with a high affinity for the HLA-G dimer, and an active glycolytic metabolism.
The dNK2 group is indicated by the presence of Annexin A1 (ANXA1) and integrin subunit beta 2 (ITGB2) proteins, which express the activating receptors of NK cell Group 2 isoform C (NKG2C), NKG2E, and the inhibitory receptor NKG2A. The dNK3 group may express T-cell immunoreceptor with Ig, CD161, immunoreceptor tyrosine-based inhibition motif domains (TIGIT), CD103, and ITGB2, although they do not express CD127 intrinsic lymphocyte cell markers (15, 26, 27).
2.2. Utilizing the Function of Natural Killer Cells for Healthier Pregnancies
Natural killer (NK) cell receptors (NKR), such as the killer-cell immunoglobulin-like receptor (KIR), leukocyte immunoglobulin-like receptor B (LILRB), C-type lectin heterodimer family (NKG2, including NKG2A, NKG2C, and NKG2D), and natural cytotoxicity receptors (NCR), including NKp30, NKp44, and NKp46, are responsible for regulating the activity of these cells (28). Numerous studies have shown that dNK cells interact with HLA ligands, including HLA-G, HLA-C, and HLA-E, produced by extravillous trophoblasts (EVT), to reduce the cytotoxicity of dNK cells (29). The NK cells express KIR2DL4 and immunoglobulin-like transcript (ILT) 2 for HLA-G. During HLA-G binding to the membrane, this molecule interacts with KIR2DL4, ultimately leading to the inhibition of dNK cell-mediated cytolysis and suppression of their cytotoxic effects. HLA-G also plays a role in spiral artery regeneration and fetal development. HLA-E reduces NK cell toxicity through the inhibitory receptor NKG2A/CD94. HLA-C and HLA-G interact with their receptors on dNK1 to promote trophoblast invasion, vascular remodeling, and the maintenance of a high-immune-tolerance microenvironment for the fetus (21, 30, 31).
Additionally, dNK1 cells have higher levels of cytoplasmic granule proteins, including perforin 1, granulysin, granzyme (Gzm) A, and GzmB, which provide protection against placental infection and glycolysis-related enzymes (29). The function of dNK cells in the process of trophoblast invasion is regulated by various cytokines, such as IFN-γ, TNF-α, granulocyte macrophage colony-stimulating factor (GM-CSF), TGF-β, and IL-10; chemokines, such as CXC motif ligand 8 (CXCL8)/IL-8, CC chemokine ligand (CCL) 3/MIP1a, CCL4/MIP1b, CCL5/Rantes, CXCL10/IP-10, and CXCL12/SDF-1; and angiogenic factors, such as angiopoietin (Ang) 2, placental growth factor (PlGF), epidermal growth factor, and vascular endothelial growth factor A (VEGF-A). For instance, Ang-2, TNF, and TGF-β prevent trophoblast invasion, but released CXCL8 and CXCL10 bind to their receptors on invasive trophoblasts and promote trophoblast motility (25, 27).
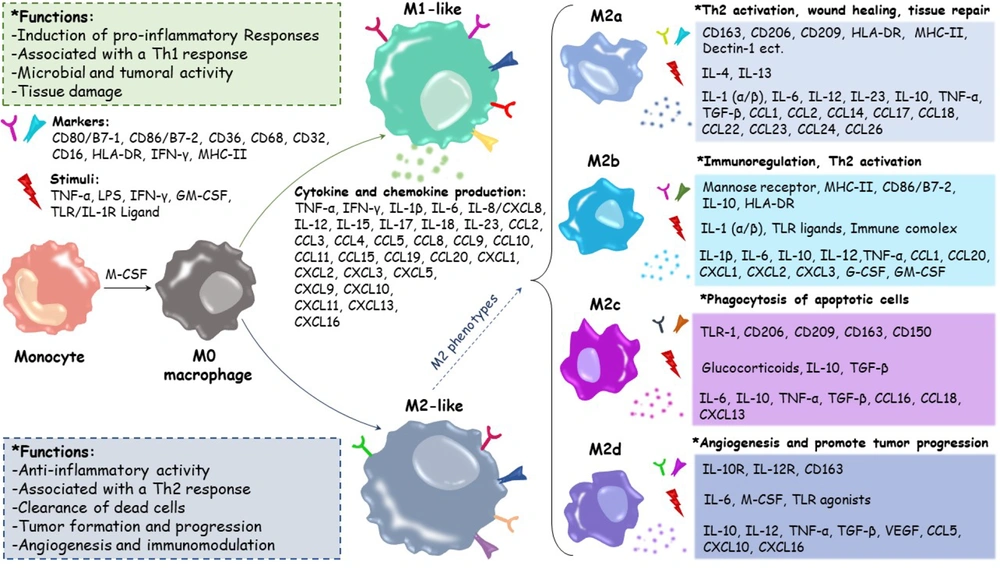
3. The Critical Functions of Macrophage Roles in Healthy Pregnancy and Fetal Development
Macrophages play a crucial role during pregnancy in healing damaged tissues and blood vessels, facilitating trophoblast invasion, and maintaining tissue homeostasis. They also serve as the primary antigen-presenting cells (APCs) in the decidual region. After NK cells, Mφs are the second largest population of decidual leukocytes. Disruption of Mφ activity and changes in their polarity (differentiation into specific phenotypes) can lead to pregnancy disorders such as recurrent spontaneous abortion (RSA), premature birth, infertility, and preeclampsia (PE), which is also associated with intrauterine growth restriction (IUGR). During the embryo implantation stage, decidual macrophages (dMφs) exhibit an M1 (proinflammatory) phenotype. Following successful implantation, EVTs invade the uterine stroma, where both M1 and M2 (anti-inflammatory) Mφs are present. The M2 phenotype is considered dominant in most dMφs to prevent embryo rejection by the immune system (32-34). This is achieved by increasing the expression of CD206, CD209, and CD163, as well as the synthesis of TGF-β and IL-10. IL-10, IL-13, IL-4, and macrophage colony-stimulating factor (M-CSF) can activate M2 Mφs (4).
The dMφs produce IL-15, which promotes NK cell proliferation. CXCL16, produced by trophoblast cells, acts as an essential molecule in establishing M2 dMφ polarity, leading to the polarization of M2 Mφs. This results in the reduction of IL-15, inactivation of NK cells, decreased cytotoxicity, and the creation of a suitable environment for embryo development (35). Other immune molecules secreted by dMφs include prostaglandin E2 (PGE2) and indoleamine 2,3-dioxygenase (36). Following a successful pregnancy, to prevent the activity of maternal T lymphocytes, the expression levels of CD80 and CD86, which are identification markers of M1 Mφs, decrease. M2a, M2b, M2c, and M2d are subgroups of M2 dMφs (34, 37).
dMφs are often derived from circulating monocytes and contribute to successful mating through the secretion of cytokines and growth factors. They also protect the embryo against various infections and create immunological tolerance for semi-allogeneic embryos. Fibroblast growth factor (FGF), VEGF-A, keratinocyte growth factor (KGF), angiogenin (ANG), Ang-1, and Ang-2 are among the factors released by dMφs that contribute to spiral artery regeneration and angiogenesis (38). The dMφs limit decidual T cells' production of IFN-γ cytokines through interactions between programmed death-ligand 1 (PD-L1)/CD274 and programmed cell death protein 1 (PD-1)/CD274, resulting in the weakening and inhibition of the immune system due to inflammation (39) (Figure 1).
3.1. The Role of HLA-G in Modulating Macrophage Function and Immune Tolerance During Pregnancy
In contrast to the conventional MHC-I molecules HLA-A, HLA-B, and HLA-C, HLA-G is a non-classical form of MHC-I that exhibits little variability. Membrane-bound isoforms are produced by transcription of HLA-G2, HLA-G3, and HLA-G4, while soluble isoforms are produced by transcription of HLA-G5, HLA-G6, and HLA-G1. Both pre-implanted embryos and EVTs express HLA-G. Soluble HLA-G (sHLA-G) is present in amniotic fluid, umbilical cord blood, maternal blood, and the culture media used for in vitro fertilization (IVF) embryos (30). KIR2DL4/CD158d, which serves as a receptor for HLA-G, is not expressed in Mφs, unlike in NK cells (21). The expression of inhibitory receptors ILT2/LILRB1 and ILT4/LILRB2 by Mφs located near invasive EVTs for HLA-G expressed by trophoblasts leads to negative intracellular signals, alters the function of secreted cytokines, and ultimately reduces the inflammatory response of the maternal immune system (34, 40, 41).
The decrease in M1 Mφ markers followed by an increase in M2 Mφ markers occurs due to the activation of Mφs by sHLA-G5, ultimately leading to an increase in the phagocytic activity of polarized Mφs. Regulating immune tolerance between the mother and fetus, as well as promoting placental growth, are important roles of this process (21). Phagocytosis of apoptotic bodies produced during the repair and regeneration of the decidual membrane and spiral artery is necessary for inducing tolerogenic immune responses, as it prevents endothelial activity and the recruitment of monocytes. Meanwhile, the phagocytic activity of Mφs against necrotic cells can lead to maternal inflammatory reactions against fetal antigens (38, 42).
Given that HLA-G expression occurs specifically during pregnancy, its vital role in reproduction, establishing immune tolerance, spiral artery regeneration, and fetal growth is well-established. Pregnancy complications caused by abnormal expression levels of HLA-G and its polymorphisms have been extensively studied in relation to their impact on pregnancy (30, 41). Polymorphisms in the regulatory regions of the HLA-G gene may influence its expression. HLA-G is highly expressed in invasive trophoblast cells of the placenta and is believed to play a role in pregnancy complications such as preeclampsia, RSA, IUGR, and preterm birth, all associated with immunological dysfunctions. These complications have been linked to low or undetectable levels of soluble HLA-G in maternal circulation (43, 44).
Current research increasingly focuses on using HLA-G as a promising therapeutic target. The dimeric form of HLA-G has attracted significant attention due to its high potential in disease treatment and improving pregnancy outcomes. However, developing effective therapeutic strategies requires a deeper understanding of the molecular and immunological mechanisms related to HLA-G and its interaction with different genotypes. Promising strategies include inducing HLA-G expression or using HLA-G-derived peptides to modulate the maternal immune response. These approaches may play a crucial role in preventing and managing pregnancy-related complications (41, 45).
4. The Role of Cytokines in Facilitating Maternal-Fetal Communication for a Healthy Pregnancy
The maternal immune system undergoes fundamental changes to protect a healthy pregnancy. Compared to the non-pregnant period, normal pregnancy is characterized by a slight increase in serum levels of both pro-inflammatory and anti-inflammatory cytokines (46). Cytokines facilitate implantation, placentation, and childbirth processes, while also maintaining maternal immune tolerance. Among the cytokines effective in successful pregnancy are IL-6 and TNF-α, which are necessary for regulating inflammatory responses. Dysfunction in cytokine expression during pregnancy can lead to complications such as PE, infection, intrauterine growth restriction (IUGR), and premature birth (1, 8, 47). Cytokines act as communication mediators between the blastocyst and the endometrium during implantation and can also support the placenta, enhance immunity, and promote the invasive and proliferative phenotypes of trophoblasts (8).
In contrast to Th2 cells, which produce anti-inflammatory cytokines like IL-10, IL-4, and IL-13 that contribute to wound healing and immunological tolerance, T helper cells (Th) type 1 are responsible for secreting pro-inflammatory cytokines IFN-γ and TNF-α (8, 48, 49). IL-1 and its family members, as representatives of pro-inflammatory factors, regulate some inflammatory diseases, including preterm birth (50). Emerging cytokines such as IL-35, IL-37, and IL-38 are involved in human pregnancy. IL-35 is an inhibitory cytokine necessary for increasing immune tolerance and preventing fetal rejection by the mother. High levels of IL-35 during pregnancy help reduce inflammatory responses and are associated with successful pregnancy. IL-37, similar to IL-35, has anti-inflammatory effects that can prevent pregnancy complications such as PE. IL-38 also regulates inflammation and potentially maintains a balanced immune environment during pregnancy (31, 51, 52).
IL-35 and IL-37 are involved in many inflammatory diseases, autoimmune disorders, malignancies, infectious diseases, and sepsis due to their anti-inflammatory and immunomodulatory effects (53). IL-35, often produced by CD4+ forkhead box protein P3 (FOXP3)+ Treg cells, was first identified in 2007. The activity of these cytokines is necessary for the suppressive properties of the Tregs population. Activated B cells, tolerogenic dendritic cells (DCs), and monocytes can produce and secrete this cytokine. IL-35 and TGF-β are responsible for increasing immunosuppressive factors in the first trimester of pregnancy, with IL-35 being the main immunosuppressive factor in the second and third trimesters. Consequently, there is a considerable drop in IL-35 levels during recurrent pregnancy loss (RPL) (51). IL-35 plays a crucial role in maintaining maternal-fetal tolerance during pregnancy. This cytokine is produced by trophoblast cells and promotes Th2 polarization, essential for successful pregnancy. IL-35 increases the production of IL-4 and IL-10 by Th cells, creating an environment conducive to fetal tolerance (54).
The IL-1 family includes IL-37, identified in 2000. This cytokine's molecular weight ranges from 17 to 25 kilodaltons, and its gene is located on chromosome 2. IL-37 expression is typically modest but significantly rises in response to inflammation (50). In normal pregnancies, baseline levels of IL-37 are consistently expressed in chorionic villous tissue and the umbilical cord (55). In patients with preeclampsia, the level of IL-37 protein in the placenta shows more than a fivefold increase compared to normal pregnancies (56). IL-38 is naturally expressed in very low amounts; however, disruption of IL-38 expression may cause many autoimmune diseases. IL-38 expression has been observed in embryonic tissues and adult tissues, such as the cardiovascular, respiratory, digestive, and reproductive systems (52). In women affected by preeclampsia, reduced levels of IL-37 and IL-38 lead to overactivation of Th17 cells and disruption of placental angiogenesis. These alterations contribute to placental dysfunction and exacerbate the disease (57-59).
5. Critical Functions of Regulatory T Cells in Supporting Healthy Pregnancy Outcomes
Regulatory T cells are a specific subset of T cells vital for building tolerance and preserving immunological homeostasis, particularly in pregnant women. Tregs function as potent immune suppressors to reduce inflammation and shield the fetus from immune system rejection by the mother. They perform these processes by suppressing the activity of effector T cells through classical mechanisms such as direct cell contact or by secreting certain cytokines (6). The FOXP3 transcription factor, identified in 2003 after extensive studies on mice (60), is the main gene for Treg differentiation, and its stable expression is characteristic of Tregs (61).
Among human Treg cell subsets, effector Treg cells (eTreg), which include CD4+CD45RA-FOXP3high, have high suppressive properties, while naïve Treg cells (nTreg), which include CD4+CD45RA+FOXP3low, possess less suppressive properties. The subset of effective Treg cells in late pregnancy is the most dominant type compared to peripheral and decidual blood Tregs (62, 63). The Treg cell count in the uterus rises dramatically in the middle to late stages of pregnancy. Late in pregnancy, there are fewer Treg cells specific to the paternal antigen in the uterine draining lymph nodes, indicating that during mid- to late pregnancy, Treg cells specific to paternal/fetal antigens migrate from the uterine draining lymph nodes to the pregnant uterus (64).
According to one study, in addition to the rise in Treg cells during pregnancy, Tregs accumulate in the uterus and draining lymph nodes each time a female mouse approaches estrus. Pregnancy-related hormones such as progesterone and estrogen, which change throughout the estrous cycle, may contribute to Treg accumulation (65). During estrus and early pregnancy, Tregs enter the uterus via chemokines such as CCL1, CCL4, CCL17, and CCL22. Approximately 70% of CD4+CD25+ Treg cells in pregnant mice express CC chemokine receptor (CCR) 5, which identifies CCL4. Additionally, the interaction of CCR8 with CCL1 can enhance the immune suppressive function of Treg cells by inducing the expression of FOXP3, IL-10, TGF-β, and the production of granzyme B (GzmB) (60).
FOXP3-HLA-G+ Tregs can express HLA-G and secrete soluble HLA-G (sHLA-G), which exerts immunoregulatory effects on a wide variety of immune cells by interacting with inhibitory receptors such as ILT2 (66, 67). Studies show that Tregs suppress NK cell cytolytic function via TGF-β (68). TGF-β also suppresses the functions of NK cells, DCs, and Mφs. The Tregs interact with DCs via CTLA-4 and LAG3 (69). Fetal trophoblast cells express and secrete several immunosuppressive molecules that play an important role in balancing Tregs (60, 70). Galectins have been shown to be important in inhibiting the maternal immune system by modifying some trophoblast regulatory processes, including promoting Treg cell development and inducing T cell death (67).
In addition to suppressing inflammatory responses to create a suitable environment for fetal tolerance, Tregs prevent complications such as spontaneous abortion and PE (65, 70). Deficiencies in the Treg population are associated with reproductive disorders, highlighting their importance in pregnancy outcomes (71).
6. Conclusions
The interaction between NK cells, Mφs, cytokines, and Tregs forms a complex network essential for establishing and maintaining a healthy pregnancy. Understanding these interactions provides valuable insights into the immunological mechanisms that support maternal and fetal health. At the maternal-fetal interface, the innate immune system—which includes NK cells and Mφs—plays a crucial role in regulating trophoblast invasion, vascular remodeling, and immunological control. Additionally, Tregs of the adaptive immune system are essential for preventing the rejection of semi-allogeneic embryos and ensuring maternal and fetal tolerance. The proper development of pregnancy depends on the cytokine-mediated balance of pro- and anti-inflammatory effects.
Selecting embryos with high HLA-G expression in IVF increases the chances of pregnancy success. Modulating regulatory cytokines or inhibiting inflammatory signals can improve dNK cell function in cases of recurrent miscarriage. The use of M2-polarization inducing factors such as IL-10 or TGF-β can reduce embryo rejection in immune infertility. Investigating future treatment strategies can lead to improvements in maternal and fetal health, increased immune tolerance, and reduced risks related to pregnancy complications.