1. Background
Malignancies of the central nervous system (CNS) are the second most common type of tumor and the most frequent type of solid tumor in childhood (1-3), which are associated with a high mortality rate (4). They are frequent in adults with a mean age of 47 years old (3, 5) and are usually caused by abnormal, uncontrolled growth of the spinal cord and brain cells, which are known as astrocytoma, meningioma, glioblastoma, ependymoma, medulloblastoma, oligodendroglioma, choroid plexus papilloma, and pineocytoma (6, 7). Although several genetic and environmental risk factors (e.g. radiation exposure, viral infections, and neuro-carcinogens) probably play important roles in the development of CNS tumors, the exact etiologies of these tumors are not well understood (8-11).
The role of viral infection in tumorigenesis of the CNS has long been under debate. However, it has been reported that some avian retroviruses can induce gliomas under in vivo conditions (12). Among human oncoviruses, herpesviruses (e.g. HCMV, HHV-6, and EBV) and polyomaviruses (e.g. SV40, JCV) have been investigated more extensively in human CNS tumors (11), which are suspected to be associated with CNS disorders. varicella-zoster virus (VZV), or human herpesvirus 3 (HHV3), is a member of the herpesvirus family that is neurotropic. Primary infection with VZV first leads to varicella (chickenpox) and, then, establishes a latent infection in the dorsal root of the ganglion. Subsequently, reactivation of the primary infection can cause zoster (shingles) as well as some complications related to the CNS (13). Only a few studies have been carried out to illustrate the role of VZV on brain tumors; therefore, there are still many gaps that need to be addressed. Furthermore, contradictory reports have been published on the frequency of VZV in brain tumors (14). Although few studies have focused on the role of VZV in CNS tumors, this hypothesis requires further investigation.
It is known that miRNAs (miR) play several critical roles in the regulation of various cellular processes, such as cell growth, differentiation, cell signaling, and apoptosis (15-17). In addition, they may act as oncomiR (a tumor inducer) or tumor suppressors in cancer cells. MiR-122, as a tumor suppressor, targets the IGF1R in breast cancer cells (18); however, it could be considered an oncomiR in renal cell carcinoma (RCC) (19). The expression levels and function of miR-122 in CNS tumors have not been well defined up to now. Several studies have shown that there are distinct expression patterns of the host miRNAs in both viral-infected and uninfected cells, wherein they can be used as novel diagnostic biomarkers (20, 21). Besides, these viruses may contribute to tumor progression by altering miRNA expression (22, 23). In this regard, a few studies have been conducted on VZV genome detection in brain tumors; so, the miRNA pattern in the VZV-infected CNS-tumor remains unevaluated.
2. Objectives
The current study aimed at evaluating the VZV infection rate in CNS tumors, as well as the expression levels of miR-122 in brain tumors.
3. Methods
3.1. Patients and Settings
The present case-control study was conducted from January 2017 to October 2019. A total of 60 CNS tumor samples were obtained from the included patients, who underwent surgery at the neurosurgery departments of hospitals affiliated with Iran University of Medical Sciences. The aim and procedures were verbally explained to all the participants, and the patients’ guardians signed the consent form freely concerning the Helsinki Declaration. In total, 45 normal CNS tissue samples as controls were obtained from a peripheral region of the surgically removed tumors from individuals matched with the case group in terms of sex and age. Patients previously treated (by chemotherapy) were excluded from the study. All the patients received dexamethasone at a dose of 8 mg/m2, which was prescribed to reduce pressure and swelling in normal tissues around the tumor before the surgery. Additionally, none of the patients were HIV-positive, and almost all of them had a WBC cell count of more than 4500 cells per µL (Table 1). Thereafter, the tumor-node-metastasis (TNM) system was used for staging CNS tumors as decided by the Department of Pathology at the same hospital. For collection, tissues are immediately preserved in RNA Later solution (Ambion, Inc., Austin, TX) and, then, stored at -80°C until the extraction of total RNA and DNA.
| Histopathology | Total (N = 60), No. (%) | Location of Tumor | Gender | Mean Age (Range) | Mean WBCa Counts Per Microliter (Range) | |
|---|---|---|---|---|---|---|
| Male | Female | |||||
| Astrocytoma | 27 (45) | Brain | 16 | 11 | 40.7 (25 - 56) | 7951.4 (4500 - 10950) |
| Glioblastoma multiform | 19 (31.7) | Brain | 7 | 12 | 61.1 (83 - 49) | 8967.5 (6190 - 14150) |
| Oligoastrocytoma | 4 (6.6) | Brain | 1 | 2 | 52.9 (20 - 74) | 7138.5 (3400 - 9100) |
| Schwannoma | 3 (5) | Meninge | 1 | 2 | 50.3 (18 - 67) | 9396.6 (6800 - 11900) |
| Meningioma | 2 (3.3) | Brain | 1 | - | 54.7 (46 - 65) | 8748.3 (7650 - 10450) |
| Pituitary adenoma | 2 (3.3) | Brain | 1 | 1 | 39.0 (35 - 44) | 10436.6 (6210 - 15490) |
| Epidermoid tumor | 1 (1.7) | Brain | - | 1 | 49.7 (37 - 60) | 9260.0 (5400 - 16700) |
| Hemangioblastoma | 1 (1.7) | Brain | - | 1 | 51.0 (48 - 54) | 7685.0 (6620 - 8750) |
| Pineoblastoma | 1 (1.7) | Brain | 1 | - | 13.5 (11 - 16) | 7700.0 (6100 - 9300) |
3.2. Nucleic Acid Extraction
The extraction of DNA was performed, using the QIAamp DNA Mini Kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer’s protocol. To evaluate miRNA, total cellular RNA was extracted from the specimens, using the Trizol approach (miRNeasy Mini Kit, QIAGEN GmbH, Hilden, Germany) following the kit’s instructions. Afterward, the achieved RNAs were converted to cDNA, using the miScript II RT Kit (Qiagen). The concentrations of the extracted nucleic acids were assessed, using a spectrophotometer, NanoDrop ND-1000® (Thermo Fisher Scientific Inc., Waltham, MA, USA) after the extraction procedure.
3.3. Polymerase Chain Reaction
For the identification of VZV-DNA, we designed primers for ORF 63 to detect all the known genotypes of VZV (Table 2). The PCR (polymerase chain reaction) assay was performed in two consecutive PCR reactions, each one containing 2.5 µL of the extracted DNA, 12.5 µL (1X) of 2x Amplicon MasterMix, and 1 µL (0.5 µM) of each primer in a 25 µL final volume. The PCR program was performed, using T100™ Thermal Cycler (Life Science Research Bio-Rad) under the following conditions: an initial denaturation step for 5 min at 95°C followed by 45 cycles (in the first PCR round) and 35 cycles (in the second PCR round) for 35 s at 95°C, for the 20 s at 58°C, and 35 s at 72°C, respectively.
| Primer | Sequence (3' → 5') | Location | TM Genomic (°C) | Target Product Size |
|---|---|---|---|---|
| First round | Orf63, 645 bp | |||
| H3F1 | GGCGGGCTTTTCACAGAA | 110334 | 60 | |
| H3R1 | CTGCGTCTGGGTGGGTTG | 110978 | 61 | |
| Second round | Orf63, 437 bp | |||
| H3F2 | CCATTGCCATTTTACCCAAG | 110482 | 58 | |
| H3R2 | TCTGGTGCGACCCATTAGAT | 110918 | 59 |
3.4. Quantitative Real-time Polymerase Chain Reaction (qRT-PCR)
Quantification of miR-122 was conducted, using the miScript SYBR Green PCR Kit (Qiagen) according to the manufacturer’s protocol. All reactions were performed in triplicate. The miRNA named SnRNA RNU6B was used for normalization in relative quantification analysis. The primer sequences used for the amplification of U6 snRNA were forward primer: 5´- CCGATAAAATTGGAACGATACAGAG- 3´ and reverse primer: 5´-TCGATTTGTGCGTGTCATCC- 3´.
Moreover, the expression levels of miR-122 were normalized to the level of U6 snRNA, as an internal control, using the efficiency-corrected calculation models of the Pfaff method (24).
The reaction mixture contained 2 µL of cDNA (0.2 - 0.5 µM), 2 µL (0.5 µM concentration) of each primer, 7.5 µL of 2 × QuantiTect SYBR Green PCR Master Mix, and nuclease-free water up to 15 µL. The optimal real-time PCR was investigated under the following conditions: In the first step, an initial denaturation was performed for 10 min at 95°C, which was followed by a further 40 cycles for 15s at 95°C, for the 30 s at 60°C, and 1 min at 72°C. The results were confirmed, using melt curve analysis that was set in the annealing/extension step. Heating was set between 50°C and 95°C at a 2°C per second rate, and continuous fluorescence measurement was, then, applied.
3.5. Statistical Analysis
SPSS version 16 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Also, multidimensional qPCR analysis was performed, using GenEx and Graphpad software. The relationship among categorical variables was calculated by running the v2 test (the Mont-Carlo method was used for the exact P-value calculation). The Kolmogorov-Smirnov test was used to assess the normal distribution of variables. Also, the independent-samples t-test/Mann-Whitney U test and the Kruskal-Wallis test were used to analyze continuous variables. In addition, Dunn's multiple comparison test and Tukey's multiple comparison test were used to estimate the correlation among quantitative variables. A P-value of < 0.05 was considered statistically significant.
4. Results
4.1. Characteristics of the Participants
In the present age-and sex-matched case-control study, we examined 45 healthy controls and 60 CNS tumor cases obtained from 29 males (mean age ± SD 48.3 ± 14.5 years old) and 31 females (mean age ± SD 47.5 ± 18.0 years old). The patients’ total mean age was 46.5 ± 13.1 years old, ranging from 11 to 83 years old, and the mean WBC count of the subjects was 8320.8 ± 2701.5 cells per µL (range, 3400 - 16700 WBC per µL). According to the histologic criteria, the primary CNS tumor samples were enrolled and, then, classified as follows: 27 (45 %) were Astrocytoma, 19 (31.7 %) glioblastoma multiform, 4 (6.7 %) Oligoastrocytoma, 3 (5 %) Schwannoma, 2 (3.33 %) Meningioma, and 2 (3.33 %) Pituitary adenoma. As shown in Table 1, in the current study, less common tumors were epidermoid tumor (no = 1) (1.7 %), hemangioblastoma (no = 1) (1.7 %), and pineoblastoma (no = 1) (1.7 %).
4.2. Determination of VZV DNA
In the present study, CNS specimens were tested for the presence of VZV ORF-63. The PCR results showed that VZV DNA existed in 13.3% of the CNS tumor specimens with a mean age of 48.8 ± 18.6 years old, ranging from 20 to 83 years. The VZV‐infected samples included Astrocytoma (5/8) and Glioblastoma multiform (3/8). There was no statistically significant association between gender and VZV positivity, as investigated by the Fisher exact test (P > 0.05).
The mean age value of the VZV-positive and VZV-negative cases was 48.8 ± 18.6 years old (ranging from 20 to 83 years) and 45.0 ± 17.3 years old (ranging from 11 to 74 years), respectively (P = 0.56). No statistically significant association was found between the presence of VZV and various types of CNS malignancies after running the v2 test, using the Monte Carlo method (P = 0.452) (Table 3).
| VZV orf63 Sequence | P-Value | |||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| Number | 7 (11.7) | 53 (88.3) | 60 | |
| Age, y | 48.8 ± 18.6 (20 - 83) | 45.0 ± 17.3 (11 - 47) | 46.5 ± 13.1 (11 - 83) | 0.56 |
| WBC counts per microliter | 9047.3 ± 3317.2 (4500 - 16700) | 8182.7 ± 2432.5 (3400 - 15490) | 8320.8 ± 2701.5 (3400 - 16700) | 0.155 |
| Tumor location | 0.2 | |||
| Meninge | 2 (28.5) | 5 (71.5) | 7 | |
| Brain | 5 (9.4) | 48 (90.6) | 53 | |
| Gender | 1.0 | |||
| Male | 5 (17.2) | 24 (82.8) | 29 | |
| Female | 3 (9.6) | 28 (90.4) | 31 | |
| Tumor pathology | 0.452 | |||
| Astrocytoma | 2 (16.7) | 10 (83.3) | 12 (20) | |
| Glioblastoma multiform | 1 (10) | 9 (90) | 10 (16.6) | |
| Oligoastrocytoma | 1 (12.5) | 7 (87.5) | 8 (13.3) | |
| Schwannoma | 1 (12.5) | 7 (87.5) | 8 (13.3) | |
| Meningioma | 2 (28.6) | 5 (71.4) | 7 (11.6) | |
| Pituitary adenoma | 1 (14.2) | 6 (85.8) | 7 (11.6) | |
| Epidermoid tumor | 0 | 3 (100) | 3 (5) | |
| Hemangioblastoma | 0 | 3 (100) | 3 (5) | |
| Pineoblastoma | 0 | 2 (100) | 2 (3.3) | |
a Values are expressed as No. (%) or mean + SD (range).
4.3. Determination of miR-122 Expression
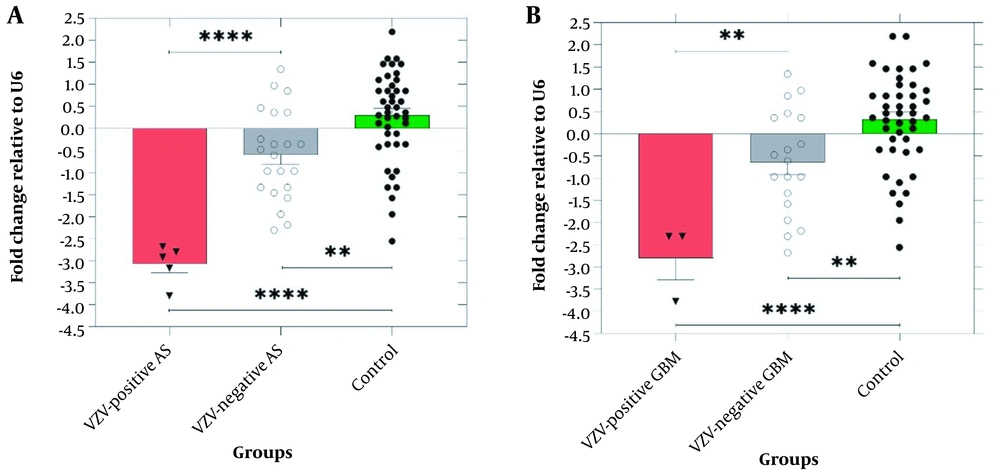
The expression level of miR-122 decreased significantly in the astrocytoma and glioblastoma multiforme samples compared with the controls (P < 0.0001) (see Table 4 for more information). The highest and the lowest expression levels of miR-122 were observed in pituitary adenoma and hemangioblastoma samples, respectively. Additionally, the expression level of miR-122 was lower in the samples infected with VZV as compared with those of the VZV-negative samples (Figure 1).
A, The expression level of miR-122 was significantly downregulated in VZV-positive astrocytoma (AS) compared to VZV-negative AS (-3.07 ± 0.22 vs. -0.59 ± 0.2, P < 0.0001) and healthy group (-3.07 ± 0.36 vs. 0.31 ± 0.19, P < 0.0001). B, The expression of miR-122 was significantly downregulated in VZV-positive glioblastoma multiforme (GBM) compared to VZV-negative GBM (-2.8 ± 0.4 vs. -0.64 ± 0.2, P = 0.0076) and healthy group (-2.8 ± 0.4 vs. 0.36 ± 0.19, P < 0.0001) (P ≤ 0.05. ** P ≤ 0.01. **** P ≤ 0.0001).
| Tumor Type | Fold-change Difference | P-Value |
|---|---|---|
| Astrocytoma (n = 27) | -1.12 ± 0.24 | < 0.0001 |
| Glioblastoma multiform (n = 19) | -1.07 ± 0.25 | < 0.0001 |
| Oligoastrocytoma (n = 4) | 0.03 ± 0.47 | Ns |
| Schwannoma (n = 3) | -0.68 ± 0.45 | Ns |
| Meningioma (n = 2) | -0.78 ± 0.65 | Ns |
| Pituitary adenoma (n = 2) | -1.5 ± 0.64 | 0.021 |
| Epidermoid tumor (n = 1) | -0.76 ± 0.9 | Ns |
| Hemangioblastoma (n = 1) | 1.33 ± 0.84 | Ns |
| Pineoblastoma (n = 1) | -1.062 ± 0.41 | 0.013 |
a Ns, not significant.
5. Discussion
In 2019, 23 820 new cases of CNS tumors and 17 760 deaths caused by brain tumors were reported in the US (7). Emerging evidence has demonstrated a frequent presence of viral infection, especially human herpesviruses, in CNS tumors in both children and adults (12, 25-30). VZV is a neurotropic virus that can cause latent infection in the dorsal root ganglia and cranial nerve ganglia (31). Moreover, the reactivation of VZV can lead to some neurological disorders, such as myelitis and encephalitis (32, 33). Therefore, the investigation of the relationship between this virus and gliomagenesis or other CNS tumors is of particular importance.
Among the 60 tumor samples examined in the present study, the sequences of VZV open reading frame (ORF) 63 were detected in 8 (out of 60) tissue samples (13.33%), including astrocytoma (n = 5/27) and glioblastoma multiform (n = 3/19), whereas no VZV-DNA was detected in the control samples. However, the statistical analysis demonstrated no significant correlation between the frequency of VZV-DNA and different tumor types. Previous studies demonstrated a higher VZV-IgG level among the normal population compared to glioma patients (34-36). These findings led to the proposal of the "neuroprotective effect" hypothesis of VZV immunoglobulin in GBM (11). However, this protective effect is mostly related to the specific immunoglobulins produced against the virus rather than the virus itself. Thus, this is not in contrast to the results of our study (11). In a study almost similar to the present study, Neves et al. (29) investigated the prevalence of herpesviruses in the cerebellum (tumor‐containing tissue) and found the virus in two tumor samples, while it was absent in the control group.
For the first time, Gelb and Dohner reported that VZV is capable of transforming mammalian cells in vitro (37). Furthermore, experimental evidence has shown that some proteins expressed by the VZV genes, such as orf-12, orf-66, and orf-63, can block apoptosis in VZV-infected cells (38-40). In another study, it was observed that ORF63 is expressed during both latent and productive infections, which promotes the survival of neurons by suppressing apoptosis, and also plays an important role in the pathogenesis of the VZV (40). These studies suggest that VZV may play a potential role in tumorigenesis.
In general, according to the studies cited, there are two hypotheses related to the role of VZV in carcinogenesis. Correspondingly, some studies reported a reverse relationship between VZV infection and brain tumors, and some others suggested that VZV can contribute to tumorigenesis through the inhibition of apoptosis.
Given the critical role of miRNAs in cellular processes, it is not surprising that viruses alter cellular conditions in their favor by dysregulation of miRNA expression (41-43). It has been observed that the expression pattern of miRNAs has been significantly downregulated in different human cancer cells, which act either as tumor suppressors or as oncomiR. Besides, several miRNAs are deregulated in various human CNS tumor cells (44, 45). The results of the current study showed a significant downregulation level of miR-122 in glioblastoma multiform, astrocytoma, pituitary adenoma, and hemangioblastoma (Table 4). Moreover, the expression level of miR-122 was found to be statistically significant among different tumor samples (P < 0.02).
As mentioned earlier, the expression level of tumor suppressors, miRNAs, has mainly increased in cancer cells, while oncomiRs are overexpressed in cancer cells. Notably, it has been observed that the expression pattern of miR-122 is different in various cancers (46, 47). Furthermore, it is reported that miR-122 acts as an oncomiR in renal cell carcinoma (19), and also as a tumor suppressor in gastric cancer (36), hepatocellular carcinoma (48-50), and breast cancer (18). In the present study, we also found that miR-122 expression was downregulated in CNS tumor tissue compared to that in the control samples. As a result, it may play a role as a tumor suppressor in human CNS cancer cells. The average fold change of relative expression showed that the expression level of this miRNA was significantly lower in the VZV-positive tumor samples compared to those of the VZV-negative tumor and control samples. Additionally, a statistically significant difference was found between these groups (P < 0.001). In contrast, Qi et al. (51) evaluated the dysregulation of cellular miRNA (including miR-122, 196, 269, 363, and 132) expression, using a TaqMan low-density array (TLDA) assay in the sera of non-vaccinated children with varicella-zoster infection (51). The different expression patterns of miR-122 used in this study and ours may be due to the different types of samples (serum vs tissue), different methods (qPCR vs TLDA), and different disease types (CNS tumor vs varicella-zoster infection) studied.
In conclusion, the present study showed that the prevalence of VZV infection in CNS tumors was 13.33%. However, more studies should be carried out to explain the roles of viral infections in these types of tumors. The expression level of miR-122 has been significantly downregulated in both tumor specimens and VZV-infected patients. Given that VZV infection may be involved in the dysregulation of miR-122 expression and since miR-122 is probably a tumor suppressor miRNA in CNS tumors, VZV may contribute to tumor progression through the downregulation of miR-122. However, the experimental data are not enough to be conclusive in this regard; therefore, further investigations are needed.