1. Background
Gestational trophoblastic diseases (GTD) are referred to as the group of heterogeneous neoplastic disorders with high blood HCG levels. The severity of various types of GTDs are different and they have different potential regarding local invasion and metastasis (1, 2). The incidence rate of GTDs is rare in western countries and, according to global statistics, it is around 0.57 to 1.2 per 1000 pregnancies; whereas, in the southeast of Asia, the annual incidence of the disease is 7 to 10 times higher than the reported values in European countries and the USA (1-3).
The clinical prognosis of the disease is strongly associated with early diagnosis and the received clinical care. Gestational trophoblastic diseases (GTD) is curable in presence of early diagnosis and appropriate treatment. However, it could lead to death and a high mortality rate in metastatic cases (4, 5). In most developed countries, GTD is regarded as a curable health circumstance as almost all patients will be recovered from the disease. However, when cancer spreads outside of the uterus, the survival rate is decreased by 20% to 30% (6). Several attempts have already been developed for early diagnosis and differentiation of GTD different pathologies and the result is promising regarding immunohistochemical methods. According to previous studies, several biomarkers like P53, P57, P63, C-erB-2 (7-10), and maspin (11) have been investigated for such purposes and it has been shown that there was a strong association between P53 and C-erbB-2 expression and trophoblastic neoplasia (8). It was also illustrated that these two biomarkers together had high negative predictive values regarding the management of molar pregnancies (8). The efficacy of immunohistochemical assessment might be an area of controversy that should be considered (12).
2. Objectives
In the current study, we aimed at investigating the association between overexpression of TP53 and HER-2/neu genes and GTD determining their predictive values in this regard. We also aimed at assessing the usage of TP53 and HER-2/neu genes as a predictor of disease progression and detecting malignant cases of GTD.
3. Methods
We performed a cross-sectional study on all 62 patients, who were referred to Imam Hossein Hospital in Tehran, Iran, and diagnosed with GTD for 5 years between 2012 and 2017. GTD was diagnosed through pathologic investigation on samples that were taken from the placenta. We also retrieved demographic and clinical information regarding age, gestational age, number of pregnancies, previous miscarriage, hypertension in pregnancy, vaginal bleeding, and history of molar pregnancy. The expression level of P53 and HER2 was assessed, using immunohistochemical approaches. The participants were categorized into 4 groups, including partial mole, complete mole, invasive mole, and choriocarcinoma according to clinical diagnosis and pathologic information.
3.1. Statistical Analysis
We described continuous variables, using mean and standard deviation, while for categorical variables, frequency number of proportion was provided. The chi-square was used to assess the association between dichotomous variables. The one-way ANOVA test was applied to find significant differences between the means of 3 or more independent groups. We also draw a receiver operating characteristic curve (ROC) to determine the best cut-point of P53 to classify invasive and non-invasive mole groups. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), area under curve (AUC), and the associated CI were also calculated. All statistical analysis was performed, using SPSS ver. 21.0. All P-values < 0.05 were considered significant.
3.2. Ethics Approval
This study is approved under the ethical approval code of IR.SBMU.MSP.REC.1398.065. The reference webpage for the ethics code number is addressed as: ethics.research.ac.ir/EthicsProposalView.php?id=60957. All participants were informed about the research objectives and completed informed consent.
4. Results
The study was performed on 62 patients with confirmed GTD, who have been referred to Imam Hossein Hospital in Tehran, Iran between 2012 and 2017. The participants were investigated in 4 groups based on their GTD status. The most frequent type of GTD was partial mole (51.6%), followed by complete mole (38.7%), and invasive mole (6.4%). On the other, the least prevalent type of GTD was choriocarcinoma (3.2%) (Figure 1). The mean (SD) of age in a partial, complete, invasive molar, and choriocarcinoma was 27.7 (6.3), 30.2 (10.9), 32.7 (11.9), and 37.2 (2.8), respectively. Overall, the proportion of abortion history was 24.1% and 4.8% of patients had a history of molar pregnancy and positive HCG in the first year was pretty low and observed in 3.2% of all study participants (Table 1).
| Characteristics | Values |
|---|---|
| Primary diagnosis | |
| Partial mole | 32 (51.6) |
| Complete mole | 24 (38.7) |
| Invasive mole | 4 (6.4) |
| Choriocarcinoma | 2 (3.2) |
| Age (Mean ± SD) | 29.6 ± 8.5 |
| Abortion | 15 (24.1) |
| Positive HCG | 3 (4.8) |
| History of molar pregnancy | 2 (3.2) |
Participants' Baseline Characteristics a
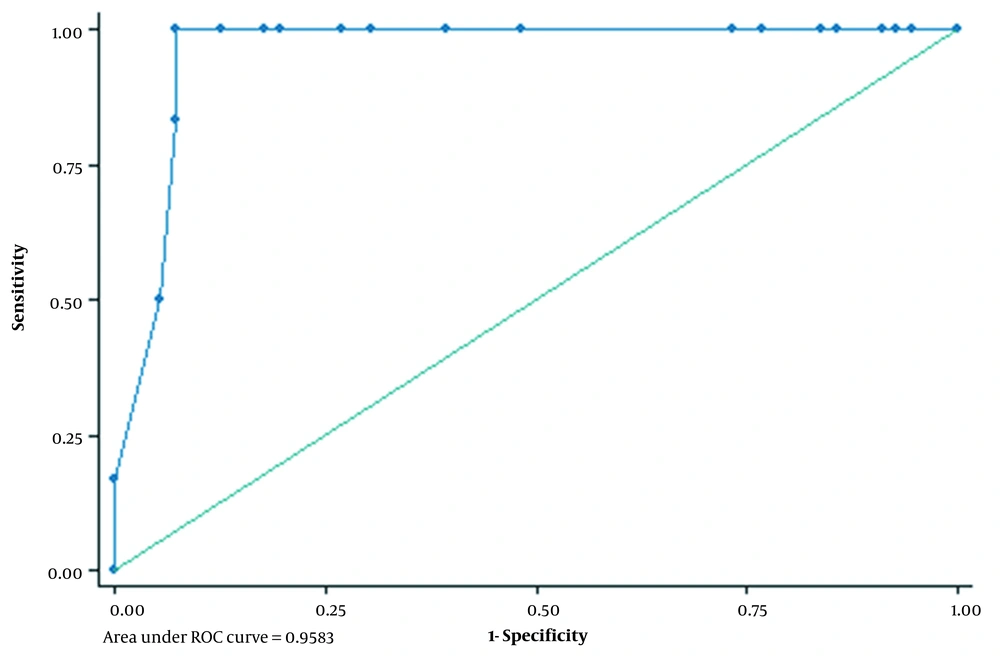
In the current research, we performed a cross-sectional analysis to investigate the association between mutation of P53 and Her-2/neu and invasion level of hydatidiform mole. We also assessed predictive values of P53 and Her-2/neu as biomarkers for diagnosis of invasive molar pregnancy. We compared the mean of TP53 over the investigated groups. According to our data, the average TP53 in partial and complete molar groups was 22.0 (18.0) and 34.7 (18.2), while it was 65.7 (2.9) in the invasive group and 74.0 (5.6) in choriocarcinoma patients. One-way ANOVA illustrated a statistically significant difference among the compared groups regarding the average expression of TP53 (Table 2). We also compared HER2 between the group and observed higher values in invasive molar (1.5 ± 1.2) and choriocarcinoma (2.5 ± 0.7) in comparison to partial (0.8 ± 0.8) and complete molar (1.0 ± 0.8) groups and the observed difference was statistically significant (P = 0.053) (Table 2). We draw a ROC curve to estimate the best cut point for P53 to differentiate between partial/complete molar from invasive molar/choriocarcinoma. According to our analysis, the best cut point of P53 was 61.0. AUC in this cut point was 0.958 (95% CI = 0.93, 0.99) (Figure 1). Sensitivity and specificity was 100.0% (54.1%, 100%) and 92.9% (82.7%, 98%), respectively. We also estimated positive and negative predictive values that were 58.1% and 100%. Sensitivity, specificity, PPV, and NPV for Her-2/neu was 66.7%, 69.6%, 17.8%, and 95.5%, respectively (Table 3).
| Gene | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| P53 | 100 (54.1, 100) | 92.9 (82.7, 98.0) | 58.1 (36.3, 77.1) | 100 (100, 100) |
| Her-2/neu | 66.7 (22.3, 95.7) | 69.6 (55.9, 81.2) | 17.8 (8.3, 34.1) | 95.5 (90.3, 97.9) |
Sensitivity, Specificity, Positive, and Negative Predictive Values of P53 and Her-2/neu in the Diagnosis of Invasive GTDs
5. Discussion
The GTD spectrum is generally accompanied by a good prognosis. However, invasive behavior of the tissues may lead to malignant transformation and metastasis. Early detection is associated with less economic burden on the health system, and higher rates of favorable pregnancy outcomes in women with limited fertility windows. Moreover, timely diagnosis may facilitate the appropriate recommendation of prophylactic chemotherapy in the high-risk group of GTN (13). Therefore, addressing a predictive marker for diagnosis of the disease progression has great importance. Several attempts have been performed to introduce a precise predictor for its development and a couple of biomarkers have shown interesting and promising results (7, 8, 11). We found that P53 was associated with a significant tendency to invasion and metastatic behaviors. The average expression of P53 was considerably higher in choriocarcinoma and invasive molar cases compared with the other forms. These malignant and invasive subtypes were more likely to have extremely high values of P53. P53 is a tumor suppressor gene that has a critical role in cell normal growth. Mutation of P53 has been shown as a risk factor in the pathogenesis of many human neoplasms and cancers (14, 15). Several studies have highlighted the overexpression of P53 in GTDs (8, 9, 11, 12). Although the results of previous research are supporting immunohistochemical methods for diagnosis and differentiation of GTD, there are some controversial findings. For instance, Ali et al. indicated that overexpression of C-erbB-2 and P53 is not informative enough regarding chemotherapy administration in patients with molar pregnancy and it seems more further studies are required (12). We reported high positive and negative predictive values for P53 regarding diagnosis of malignant progression of molar pregnancy. Such findings have also been found by Hasanzadeh et al. that reported a positive predictive value of 90% and negative predictive value of 92% for p53-positive cytotrophoblasts (8). Our findings were also concordant with Chen et al., demonstrating that expression of P53 may serve as an effective diagnostic instrument in the prediction of metastatic molar pregnancy (9). Fayed et al. have also shown higher expression of P53 in the invasive molar pregnancy group compared with the non-invasive molar group, which is in line with the results of the current study (10). Such a positive correlation between the expression of P53 and GTNs has also been reported by Sun et al. (11).
In the present study, the proportion of positive Her-2/neu was 33.8% observed in 21 patients. The frequency of Her-2/neu positive phenotype in the invasive group was 66.6%, while it was 30.3% in the non-invasive group. We did not observe any significant association, which might reflect our small sample size and modestly insufficient statistical power. The sensitivity of Her-2/neu for diagnosis of invasive cases was pretty low, while we reported high specificity. Our results are supported by Menczer et al. that indicated the same findings for Her-2/neu overexpression in the prediction of GTN (13). According to their findings, the sensitivity of Her-2/neu expression for predication of GTN was 22.2%, while specificity was 83.3%, which is consistent with our values (13). They also showed a relatively good negative predictive value (74.1%), despite a very low positive predictive value of 33.3% (13). Another study depicted that expression of Her-2/neu was significantly higher in choriocarcinoma cases compared with partial mole and complete mole groups (16). These findings could not be compared with our data as we had a limited number of choriocarcinoma cases and it was impossible to address the statistically significant difference. However, the average expression of Her-2/nu in 2 cases of choriocarcinoma in our study was partially higher than in other groups, which warrants further studies in this regard.
The current study is one of the limited attempts to address the correlation between Her-2/neu expression and hydatidiform moles. We also applied one of the most precise immunostaining approaches. However, our findings must be interpreted in the context of our limitations. Lack of statistical power due to the small sample size particularly in invasive molar and choriocarcinoma was the main limitation of the current study that could affect our findings. Hence, further studies with larger sample sizes might be required.
5.1. Conclusions
To sum up, overexpression of P53 might be considered a potential biomarker to predict the progression of GTD toward malignancy. However, replication of these results in subsequent studies is required to confirm the clinical benefits. On the contrary, due to the low sensitivity and positive predictive value of Her-2/neu, the utility in differentiation between invasive and non-invasive GTDs is uncertain.