1. Background
Gastric cancer is the fourth most common type of cancer and the second leading cause of cancer death worldwide (1). Developing this disease in humans is a multi-stage process which mainly caused by infectious, environmental and genetic factors (2, 3). Helicobacter pylori infection, lifestyle, and diet are among the environmental risk factors of gastric cancer, while mutations and polymorphisms are the main genetic factors associated with this cancer. These genetic determinants exert their impact by altering the expression or function of proteins (4). Moreover, polymorphisms influence the susceptibility of a person to diseases or response to drug therapy, and are therefore recognized as important factors in personalized medicine (5).
Increased levels of prostaglandin (PG) have been observed in patients with cancer, which could play an important role in cancer progression and metastasis (6, 7). Producing PG depends on the activation of cyclooxygenase (COX), which transforms arachidonic acid into eicosanoids, including PG. There are 2 forms of COX enzymes: COX-1 is continuously expressed in most tissues, while COX-2 is rapidly induced as part of inflammatory responses to extracellular stimuli and plays an important role in the regulation of cell proliferation, differentiation, and carcinogenicity (8, 9). Previous studies have shown increased levels of COX-2 in transformed cells and various cancer cells (10, 11). The increased amount of COX-2 may be involved in reducing intracellular free arachidonic acid, which subsequently prevents apoptosis (12, 13). COX-2 also increases the ability of cancer cells to invade neighboring tissues (14, 15). Studies have shown that the COX enzymes, particularly COX-2, play an important role in the development of gastrointestinal cancer (12, 16). In addition, some studies demonstrated a direct relationship between COX-2 expression and the development of cancer (17, 18).
2. Objectives
Given the controversial role of COX-2 gene polymorphism and its expression in gastric cancer, this study investigated polymorphism of the COX-2 gene and the amount of the COX-2 enzyme in patients with gastric cancer. The findings of this study could be useful for controlling and treating this type of cancer.
3. Methods
In this case-control study, 150 patients with T-stage gastric cancer (average age: 62.14 ± 12.66 years) were admitted to hospitals affiliated with the Golestan University of Medical Sciences in Gorgan (Iran) between 2018 and 2020. The sample size was determined based on the desired accuracy with a confidence level of 95% source (P = 50%). With the help of physical and endoscopic examinations, diagnosis of stomach cancer was made based on the International Standard Classification of Diseases for Oncology IX, code 151, Lauren criteria (19), and was later confirmed by a pathologist. In addition, 150 healthy individuals (average age: 58.93 ± 14.2 years) were selected as a control group. They matched with the patients in terms of age, gender, region, and race were selected as controls. The subjects had no history of systemic diseases and gastrointestinal system diseases such as pancreatic pseudocyst, biliary dyskinesia, and hepatitis. The GI mucosa in these individuals appeared visually appeared normal with endoscopy. The study procedures were performed in accordance with medical ethics standards. Demographic data were collected using a questionnaire, and 10 mL of venous blood were taken from all subjects. Genomic DNA was extracted using a modified method of protein precipitation at high salt concentrations. First, the DNA extraction solution (DNG-Plus kit, Cinnagen, Iran) was placed at 37°C for 20 minutes. Then, 100 μL of the purified sample was mixed with 400 μL of the DNG-Plus solution. After homogenization, the samples were dissolved in isopropanol and 75% ethanol. The mixture was centrifuged and the DNA-containing supernatant was transferred to a separate tube. The purity of the extracted DNA was assessed by reading absorbance using a spectrophotometer (OD 260/280). Primers were designed based on the polymorphism in the promoter region of the COX-2 gene (-765 G → C), using the NCBI, and Eukaryotic Promoter Database (EPD) websites. The primers were then synthesized by CinnaGen Co., Iran (Table 1).
| Length | Sequence (5'-3') | %GC |
|---|---|---|
| 25 | Forward 5'- GTCCATCAGAAGGCAGGAAACTTTA -3' | 44 |
| 25 | Reverse 5'- TGTCTGGTCTGTACGTCTTTAGAGG -3' | 48 |
Sequence of the Primers Used in the Study
Amplification of the desired region was performed using PCR. A 406 bp fragment was the anticipated polymerase chain reaction (PCR) product. Final PCR solution (25 μL) contained 150 ng of genomic DNA, 2.5 μg of PCR buffer, 2.5 mM MgCl2, 200 nmol dNTP, 200 nmol of each primer, 0.3 μmol of Tag polymerase, and dH2O. The PCR cycling conditions were as follows: initial denaturation at 94°C for 3 min, 30 cycles of denaturation at 84°C for 30 sec, annealing at 58°C for 45 sec, extension at 72°C for 45 sec, and final extension at 72°C for 12 min. The PCR products were subjected to electrophoresis on 5% agarose gel.
Restriction fragment length polymorphism-PCR (RFLP-PCR) was used for detecting polymorphisms. The PCR products were digested with appropriate restriction enzymes, and the presence or absence of the restriction sites was confirmed by electrophoresis. For the restriction enzyme digestion, 2.5 units of the enzyme AciI (identifies and cuts G allele in the G/C polymorphism region) and 1 μL of enzyme buffer were incubated at 37°C for 16 hours. The products were separated by electrophoresis on 2% agarose gel. Using the genotypes derived from RFLP, frequencies of the C and G alleles in the promoter region were calculated.
The activity of the COX-2 enzyme was evaluated by spectrophotometry at 270 nm at 37°C. Data were analyzed using SPSS software (version 20). Chi-square and t-test were utilized to investigate the relationship between COX-2 gene polymorphism and COX-2 activity. The frequency of genotypes and alleles following COX-2 polymorphism was evaluated using direct observation and count of amplified gene fragments. After verifying the normality of data distribution using the Kolmogorov-Smirnov test, quantitative data were analyzed using an independent t-test and qualitative data were analyzed using the chi-square test or Fisher's exact test. The relationship between gene polymorphism and disease and its various stages was investigated using logistic regression analysis, and the odds ratio was measured at a confidence level of 95%.
4. Results
Based on the results, family history of gastric cancer was positive in 29 (19.33%) patients and 13 (8.66%) control individuals. Moreover, the 2 groups significantly differed in terms of risk factors and positive history of H. pylori infection (Table 2).
| Variables | Controls | Cancer Patients | P-Value |
|---|---|---|---|
| Risk factors | 0.04 b | ||
| Smoking | 20 (13.33) | 22 (33) | |
| Consumption of hot drinks | 38 (25.33) | 76 (50.66) | |
| Consumption of salted fish | 15 (10) | 16 (10.66) | |
| Consumption of fast food | 41 (27.33) | 29 (19.33) | |
| Consumption of pickled food | 100 (66.66) | 88 (50.66) | |
| Gender | 0.36 | ||
| Male | 74 (49.33) | 77 (55.33) | |
| Female | 76 (50.65) | 73 (48.66) | |
| Helicobacter pylori infection | 0.00 b | ||
| Yes | 32 (21.33) | 76 (50.66) | |
| No | 118 (78.66) | 74 (49.33) |
Frequency Distribution of Demographic Characteristics in Patients with Gastric Cancer and Healthy Individuals a
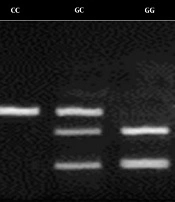
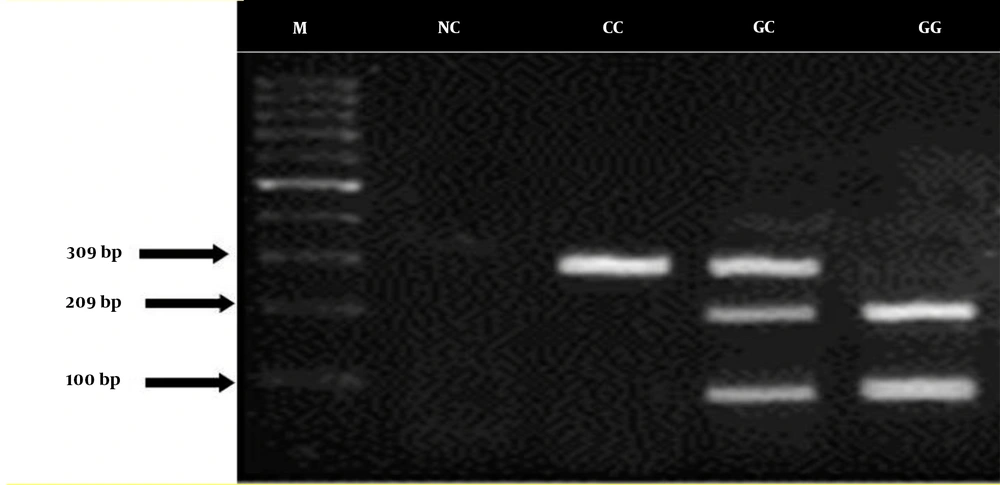
In this study, we observed a 309-bp band for homozygous CC, 3 bands of 100, 209, and 309 bp for heterozygous CG, and 2 bands of 100 and 209 bp for heterozygous GG (Figure 1).
The results showed that the G-765C polymorphism is related to the risk of gastric cancer, and there was a significant difference in the frequency of homozygous CC genotype between the patients with cancer and healthy controls. In patients with gastric cancer, the frequency of CC, CG, and GG genotypes was 23.33%, 50.66%, and 26%, respectively. In the healthy subjects, the frequency of CC, CG, and GG genotypes was 27.33%, 60%, and 12.66%, respectively. Overall, the frequency of GG genotype and CC genotype was significantly higher in patients with gastric cancer and healthy individuals, respectively (P < 0.05). However, the frequency of CC genotype did not differ significantly between the 2 study groups (Table 3).
| C/C | C/G | G/G | |
|---|---|---|---|
| Gastric cancer | 35 (23.33) | 76 (50.66) | 39 (26.00) |
| Healthy individuals | 41 (27.33) | 90 (60.00) | 19 (12.66) |
| P-value | 0.0932 | 0.0312 | 0.004 |
Frequency of CC, CG and GG Genotypes in Patients with Gastric Cancer and Healthy Individuals a
5. Discussions
COX, also known as PG-endoperoxide synthase, is a key enzyme in the conversion of arachidonic acid to PGs, which is associated with inflammation, pain, angiogenesis, cancer, and Alzheimer's disease (9). In the present study polymorphism of the COX-2 gene and amount of the COX-2 enzyme in patients with gastric cancer were confirmed. Numerous studies have shown elevated levels of COX-2 in transformed cells and various cancer cells (14, 20). Elevation of COX-2 has been also observed in the cartilage of patients with osteoarthritis and the joint tissue of patients with rheumatoid arthritis. On the other hand, anti-inflammatory cytokines such as IL-4 and IL-13 as well as glucocorticoids, reduce COX-2 expression, which can be effective for managing inflammation. It has been revealed that regular use of aspirin or other non-steroidal anti-inflammatory drugs (NSAIDs) reduces the risk of developing colorectal cancer by 40 - 50%. Studies on animal models of colon cancer also demonstrated that the use of NSAIDs significantly reduces the number of tumors. Preliminary observations in this regard have revealed the presence of high COX-2 levels in colorectal tumors, while the amount of the enzyme is negligible in natural gastrointestinal mucosa (9). The relationship between COX-2 levels and the risk of cancer has been less extensively investigated in patients with gastric or esophageal cancer. Researchers produced transgenic mice capable of overexpressing the human COX-2 gene, especially in the mammary gland (21). This caused a high rate of hyperplasia, dysplasia, and mammary gland transformation in female mice, which indicates the role of COX-2 expression in tumor induction. Furthermore, COX-2-knockout mice had a 75% lower risk of developing chemically-induced skin papillomas. According to pharmacological evidence, selective COX-2 inhibitors, such as celecoxib and rofecoxib could reduce tumor formation in the tongue, bladder, lung, skin, breast, and intestine of animal models (14). Another study also reported that COX-2 knockout reduces the number and size of intestinal polyps (22). Biramijamal et al. reported an increased level of COX-2 in Iranian patients with esophageal squamous cell carcinoma, which was also accompanied by mutation in the p53 gene (20). Increased level of COX-2 is also associated with anti-apoptotic effects and increased VEGF production and angiogenesis, all of which contribute to tumorigenesis and progression to metastasis (12). However, the effects of COX-2 overexpression in colon cancer can be reversed by selective COX-2 inhibition with NS-398 (14). In this study, we found that the GG genotype of COX-2 was significantly more frequent in patients with gastric cancer compared to healthy individuals. A study by Hafez and Tahoun in Egypt found a positive relationship between COX-2 overexpression and gastric cancer (18). In a study on 100 patients with cancer and 150 healthy individuals reported the positive relationship of COX-2-765G/G genotype, alcohol consumption, and smoking with risk of gastric cancer (23), which is in line with our findings. In a similar study in Turkey, COX-2 expression was positive in 16 (61%) tumor samples and negative in 6 (23%) tumor samples, but the overall expression of the enzyme in patients with gastric cancer was higher in tumor tissues than in tumor-adjacent tissues (24). In another study in Iran, older age and CC genotype in women were significantly associated with the risk of developing gastric adenocarcinoma (25), which is inconsistent with our findings. COX-2 is responsible for the production of PGs in response to internal and external stimuli.
5.1. Conclusions
Based on the results, it can be concluded that inhibition of COX-2 might be effective for preventing or treating cancer. Nevertheless, further studies should be carried out on the mechanism of action of COX-2 inhibition in cancer treatment.