1. Background
Inhalation of particulate matter (PM) from fossil fuel combustion is associated with adverse health effects, including reduced lung function (1) and increased mortality (2). Although the mechanism for PM-induced health effects is not fully defined, animal models and in vitro studies suggest that pro-inflammatory cytokine release from airway cells is an important factor (3). Inflammation may play a role in the etiology of lung cancer. Environmental agents associated with elevated lung cancer risk, such as ambient particulate matter, may damage the lung by inducing chronic inflammation. Lung cancer risk is elevated in individuals with emphysema (4, 5), interstitial lung disease (6), and asthma (7), which could similarly reflect effects of the underlying inflammatory disorders.
Induction of pro-inflammatory mediators by alveolar macrophages exposed to ambient air particulate matter has been suggested to be a key factor in the pathogenesis of inflammatory and diseases in the lungs. However, receptors and mechanisms underlying these responses have not been fully elucidated. Different contributing physiological and psychosocial factors have been proposed (8). A few prior studies have examined lung cancer risk in relation to polymorphisms in the genes coding for inflammation pathway signaling molecules, such as Interleukin 1β (IL-1β) (9-11), IL-1 receptor antagonist (IL-1RN) (12, 13), IL-6 (10, 14), IL-10 (15), cyclooxygenase 2 (14), and tumor necrosis factor-α (16). These inflammatory cytokines are regulated by the pro-inflammatory transcription factor, nuclear factor NF-κB (8).
Given the close interaction between the external environment and the lung, TLRs have been implicated in lung-associated immune responses, including airway hyper responsiveness (AHR) and allergic asthma (17). Dysfunction and unregulated activation of the TLR pathway can contribute to decreased lung function and the pathogenesis of acute and chronic lung inflammatory diseases (18). TLR activation, can occur via two pathways: 1- the Myeloid Differentiation primary-response protein 88 (MyD88)-dependent pathway, and 2- the MyD88-independent pathway. These two pathways correspond to early and late-phase NF-κB signaling and pathway-specific induction of pro-inflammatory cytokines and chemokines (19, 20). Inflammation play an important role in the etiology of lung cancer.
Regular aerobic exercise results in multiple health benefits, including improvement of cardiorespiratory fitness and quality of life, reduction of obesity and blood pressure, and increased longevity (21, 22). When performed chronically on a regular basis, aerobic exercise also reduces oxidative stress systemically (23) in different diseases, such as heart diseases, type 2 diabetes, rheumatic arthritis, and alzheimer and parkinson diseases (23) , as well as in the airway epithelial cells of animals with long-term allergic lung inflammation (24). Chronic practice of regular exercise exerts a marked anti-inflammatory effect in different models of pulmonary diseases, such as in asthma models (25-28), acute respiratory distress syndrome (29, 30), and chronic obstructive pulmonary disease (31).
Studies that have investigated the effects of exposure to air pollutants during exercise have suggested that people exercising in polluted environments are at increased risk of respiratory and cardiovascular morbidity related to air pollution owing to an exercise-induced amplification in respiratory uptake, lung deposition, and toxicity of inhaled pollutants (32-35). Exercise may increase the likelihood of an adverse effect by increasing the dose of pollutants delivered to target sites in the lungs as ventilation increases to meet metabolic demands (36). However, these studies do not take into account the potential anti-inflammatory and health effects of exercising in air pollution (37), which could inhibit the pro-inflammatory events induced by air pollution.
2. Objectives
Therefore, the aim of this study was to investigate the effects of 4 weeks of aerobic exercise performed in association with carbon black PM10 exposure on lung tissue inflammation and lung cancer.
3. Materials and Methods
In all experiments, the Tarbiat Modares university guidelines for animal care was followed. This study was approved by the Tarbiat Modares University of Tehran (code number: 62.2987).
3.1. Animals
Twenty four adult male Wistar rats aged 8 weeks were obtained from Pasteur Institute of Iran and randomly divided into the 4 groups: A; control (without exposure carbon black PM10 and aerobic exercise; n = 6), B; aerobic exercise (five times per week for 4 weeks; n = 6), C; exposure to carbon black PM10 (5 mg/m3; per rat; n = 6), D; aerobic exercise concomitantly with exposure to carbon black PM10 (n = 6). Rats were housed in cages under controlled environment (23°C and 12 hour light-dark cycle) with free access to normal chow and tap water.
3.2. Exposure to Carbon Black PM10
Figure 1 shows the inhalation chamber at the laboratory of Tarbiat Modares university (Falonak) where inhalation exposure was carried out. Carbon black dust (38) obtained from Iran-carbon factory. Rats in groups of C and D were exposed to carbon black in the inhalation chamber at nominal concentrations of 5 mg/m3 for 2 h/day, 5 days per week for a total of 4 weeks. The control rats were exposed to clean, filtered air containing no carbon black for the same period. The concentrations, size and shape of CB particle were monitored once time weekly by Grimm Aerosol Technique (GmbH and Co. KG. Dorfstraße 9 - 83404 Ainring-Germany (and light microscope (Acc.V-Spot magn. 25.0 KV 3.4. 5000 x), respectively.
3.3. Exercise Treadmill Test and Training
Animals in B and D groups were adapted to the treadmill for rat (will running treadmill, Lafayette American) training for 3 days (15 minutes, 20 m/min). On the fourth day, the individual maximal exercise capacity test was performed with a 5-minute warm-up (6 m/min) and followed by an increase in treadmill speed (3 m/min every 3 minutes) until animal exhaustion (i.e. when they were not able to run voluntarily after 3 mechanical stimuli) (24, 27, 28). The maximal exercise capacity (100%) was defined as the maximum speed reached by each animal. The physical test was performed for each rat individually. The speed average of each group was calculated, and then the rats were submitted to treadmill training as a mean speed of the group workload. rats were trained at low intensity, corresponding to 50% of the initial maximal speed obtained in the exercise test, for 60 minutes, five times per week, as previously described (24, 27, 28).
3.4. Analysis of mRNA Expression TLR4, NF-κB and TNF-α by RT-PCR
After sacrificed rat and total lung tissue, RNA was isolated using Trizolereagent (Qiagen, Germany), according to the manufacturer’s instructions. The RNA samples were subjected to reverse transcription using thermo scientific revert aid first strand cDNA synthesis kit (Ferementase). In the subsequent step, the cDNAs were used as templates to perform real-time PCR using SYBR green PCR master mix (SYBR green I,) by step one ABI system (Applied Biosystem). The crossing threshold values assessed by the real-time PCR were evaluated for the transcripts and normalized to the results for GAPDH mRNA. The corresponding primer pairs for TLR4, NF-κB, and TNF-α and GAPDH (housekeeping gene) were listed in Table 1.
| Gene | Primer Sequence |
|---|---|
| Forward | 5´-AATCCCTGCATAGAGGTACTTCCTAAT -3´ |
| Reverse | 5´-CTCAGATCTAGGTTCTTGGTTGAATAAG -3´ |
| Forward | 5´-AACACTGCCGAGCTCAAGAT -3´ |
| Reverse | 5´-CATCGGCTTGAGAAAAGGAG -3´ |
| Forward | 5´-GACCCTCACACTCAGATCATCTTC -3´ |
| Reverse | 5´-TGCTACGACGTGGGCTACG -3´ |
| Forward | 5´-GACATGCCGCCTGGAGAAAC -3´ |
| Reverse | 5´-AGCCCAGGATGCCCTTTAGT -3´ |
3.5. Real-Time PCR
All of the tests were repeated two times in each group. The threshold cycle (Ct) for each specific gene, corresponding housekeeping gene (GAPDH) and their differences (ΔCt) were determined and then evaluated gene expression changes using 2-∆∆CT formula.
3.6. Statistical Analysis
Results are expressed as Mean ± SD. Differences in body weight between pre and post interventions were examined by 2-tailed t-test. In order to determine the significant differences between groups one way ANOVA and LSD post hoc test and Kruskal-Vallis test after examined normal distribution of data by Kolmogorov-Smirnov test were used. P < 0.05 were considered statistically significant.
4. Results
Body weight: There were significant differences among the A (control) and C (PM10 exposure only) groups in body weight pre and post interventions. The mean of increase in body weight in C group was higher than A, B and D groups (Table 2).
| Groups | Initial Test | Final Test | Final-Initial Test | P Value |
|---|---|---|---|---|
| 285.83 ± 25.55 | 324.33 ± 27.12 | 38.50 ± 1.57 | 0.045 | |
| 279.17 ± 32.55 | 304.33 ± 43.31 | 25.16 ± 10.76 | 0.090 | |
| 270.50 ± 27.12 | 311.17 ± 36.66 | 40.67 ± 9.54 | 0.023 | |
| 281.67 ± 22.73 | 315.67 ± 33.23 | 34.00 ± 10.50 | 0.101 |
a Data are presented as mean ± SD.
b Significantly Different at P ≤ 0.05.
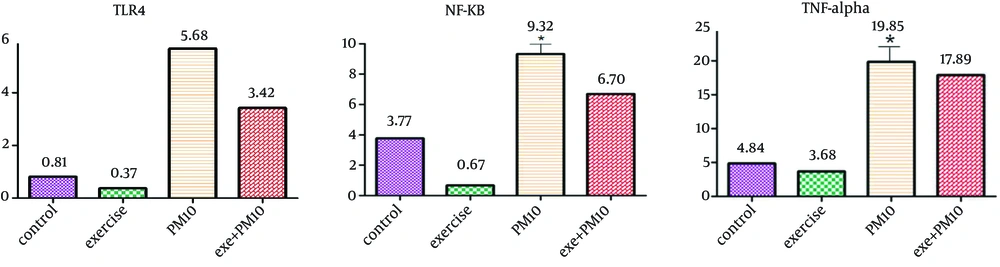
4.1. Effects of Aerobic Exercise and Carbon Black PM10 Exposure on Gene Expression TLR4. NF-κB and TNF-α
Presented data in Figure 2 demonstrate that PM10 carbon black exposure increased the gene expression TLR4, NF-κB and TNF-α in lung tissue compared with all of the other groups (P ≤ 0.05) and that aerobic exercise in carbon black PM10 exposure decreased the expression of these cytokine compared with the carbon black PM10 exposure group (P ≤ 0.05). 0ne way ANOVA also demonstrated that carbon black PM10 exposure presented a significant effect on the TNF-α (P = 0.047) and NF-κB (P = 0.014). No significant effect was observed on the gene expression TLR4 by Kruskal-Vallis test (P = 0.325).
5. Discussion
In the present study, we demonstrated that aerobic exercise inhibits lung inflammation and pro-inflammatory cytokine release in lung tissue in an experimental model of PM10 carbon black-induced lung inflammation.
Considerable epidemiological and toxicological studies have established a clear link between exposure to air pollution particles and adverse pulmonary health effects. Several experimental and human studies have demonstrated that increased levels of air pollution associated with pulmonary inflammation (37, 38). The association between ambient air pollution particles exposure and lung cancer risk has been investigated in prospective studies and the results are generally consistent, indicating that long-term exposure to air pollution may cause lung cancer.
Gene expression analysis identified the association with the effect of PM10 carbon black on the innate immune response. PM10 carbon black exposure increased the gene expression TLR4, NF-κB and TNF-α in lung tissue. TLR4 activation is corresponded to NF-κB signaling and pathway-specific induction of pro-inflammatory cytokines and chemokines or interferon signaling (39).
An extensive research in recent years has been indicated that chronic inflammation leads to various chronic disorders associated with cancer (40-42). A central role in the induction of chronic inflammation is played by a set of genes encoding pro-inflammatory cytokines such as IL-1, IL-2, IL-6, and TNF-α and monocyte chemotactic Protein 1 that are regulated by the transcription factor (NF-κB) (43-45).
Particulate matter exposure could effect on Obesity (46). These conditions are associated with derangements in the interplay between metabolic and immune processes and inflammation (47). The authors observed significant increase in body weight with PM10 carbon black exposure which could explain the increased inflammation can be caused by PM10 exposure.
Studies have suggested that potential anti-inflammatory effects of aerobic exercise could inhibit the pro-inflammatory events induced by air pollution. The anti-inflammatory effects of exercise (48-50) have focused on three possible mechanisms: the reduction in visceral fat mass; increased production and release of anti-inflammatory cytokines from contracting skeletal muscle (such molecules are termed myokines (48-51); and reduced expression of Toll-like receptors (TLRs) on monocytes and macrophages (52). In the present study, aerobic exercise in PM10 carbon black exposure decreased the gene expression TLR4, NF-κB and TNF-α in lung tissue and also body weight of animals. Although this change in gene expression was slight, however this result can be clinically important.
We conclude that low-intensity aerobic exercise presents protective effects from PM10 carbon black-induced lung inflammation. Future studies should therefore be directed towards better defining the mechanism involved in the induction of symptoms by inflammatory molecules and risk of cancer in exposure to air pollution particles.