1. Background
Malignancies are the second cause of death in developed countries, and among malignancies head and neck cancers are the six most prevalent neoplasms worldwide, with an estimated 900,000 new cases diagnosed annually. Oral squamous cell carcinoma is the most common cancer in oropharyngeal area (1-4).
Cancer treatments have been changed through the last 3 decades and include surgery, chemotherapy, and radiotherapy. Oral complications are commonly experienced by patients undergoing cancer therapy (5). Radiotherapy complications are affected by radiation dose, fraction size, volume of irradiated tissue, type of ionizing irradiation and fraction scheme (6). Radiotherapy of oral cavity can induce mocusitis which is the most common acute complication (7). Ionizing rays can cause inflammation of oropharyngeal mucosa especially in the 2nd and 3rd weeks and leads to ulcers and erosions which are so painful and cause problems in eating; therefore, cancer treatment protocol might be postponed due to the patients’ malnutrition (8, 9). Mocusitis will occure in all patients under radiotherapy (10). Age, gender, oral hygiene, radiation dose, smoking, systemic disease and radiotherapy techniques are risk factors (10). The first sign of mucositis is a white patch caused by interruption in desquamation, then mucosa with erythema is noted within 1 to 2 weeks, and throughout the course of therapy pseudomembrane formation represents ulceration which is accompanied by pain and soreness (11). Extraction of hopeless teeth, relining of dentures, and ameliorating of oral hygiene can decrease the severity of mocusitis. Although different therapy modalities such as amifostin, G-CSF, KGF, Mg milk, amphogel, kaopectate, benzydamine hcl, capcasin, sucralfate and low level laser are suggested by researchers. There is still no definite and standard way to treat or prevent this condition. Xerostomia as another radiation complication could deteriorate the condition.
Propolis is a resinous material which is made by honey bee. It can be red, brown or green depending on the plants of the area. It has a special scent which is due to fat and protein. The ingredients make propolis adhesive to skin (12). The most important part is polyphenols including galangin, luteolin, campferol, capheic acid phenyl ethyl ester (CAPE), quercetin, artipelin, vitamin, sequiterpene quinines, coumarins, amino acids, steroids and inorganic compounds and aminoacids (12, 13). Propolis is used in homeopathic and herbal practice as an antiseptic, anti-inflammatory, antimycotic, and bacteriostatic agent (14). Animal studies suggested that polyphenolic compounds have antioxidant and a free radical scavenger effects and are responsible for protecting against radiation (15). When it is administered preventively, it has higher efficacy. The results suggest that propolis and related flavonoids given to mice before irradiation protected mice from lethal effects of whole-body irradiation and diminish primary DNA damage in their white blood cells as detected by the alkaline comet assay (16).
2. Objectives
Our goal is assessing the preventing and therapeutic effect of propolis in radiotherapy induced mucositis in patients with head and neck cancer.
3. Patients and Method
This triple blind placebo controlled randomized clinical trial was conducted in Omid hospital, cancer center, Mashhad university of medical sciences.
Patients were assessed according to the eligibility requirement. 20 patients involved with head and neck malignancies including 14 (70%) male and 6 (30%) female met the inclusion criteria and participate in the study and consecutively enrolled in this program (Box 1).
| Criteria |
|---|
| Age over 15 years old |
| At least half of the mouth to be under radiation |
| Radiotherapy was administered with dose of 50-70 cGy |
| Involving with head and neck cancer and undergoing radiotherapy |
| WBC < 3000 mm3 |
| FBS > 150 mg/dL |
| If they had other serious systemic disease |
| Previously underwent radiotherapy, chemotherapy |
| Obtaining grade 3 mocusitis accordingly |
Inclusion and Exclusion Criteria
The patients could deny or cut the protocol whenever they wanted. All patients were advised to avoid alcohol, spicy and scour foods, smoking, and to maintain good oral hygiene during the course of radiotherapy. Radiotherapy was administered using Cobalt 60 radiotherapy unit at a dose of 2 Gy per day, five times a week up to total dose of 50 to 70 Gy.
The two under study mouthwashes (propolis or placebo) were provided by SORENTEC company in 3% concentration and finally packaged in identical containers labeled as A and B. 20 patients fulfilling the inclusion criteria were divided into 2 groups by completely random method treated by propolis (group A) or placebo (group B). None of the patients nor researchers was aware of the medication inside. Only pharmacist knew and prepared the corresponding drug for every patient but had no contact with study participants.
Each patient received 2 bottles of mouth wash and a bottle of normal saline for oral rinsing before using the mouthwash. All the patients were instructed to rinse their mouths with 15 ml of placebo or propolis 3% and then to swallow 3 times a day for 5 weeks simultaneously with radiotherapy protocol from the first session.
Examinations of the oral mucosa were performed on all patients at the beginning of treatment and continued weekly up to the end of the study for 5 weeks.
The national institute of the cancer common toxicity criteria oral mucositis scale was used for grading mucositis and the xerostomia was assessed by 5 standardized questions, validated by other researchers (17, 18).
All of the scores for mucositis and xerostomia were assessed and recorded at the beginning and weekly, for 5 weeks (the end of radiotherapy) by researcher who were blinded for medication.
The weight of patients was measured at the beginning and at the end of the treatment. If there was any adverse event, treatment was discontinued and the subject was referred for necessary care.
Statistical analysis was performed with SPSS 20. Data were analyzed using the independent sample t-test, Man Whitney and Friedman tests with P < 0.05 considered significant.
This project was approved by the Ethics committee of Mashhad university of medical science, Iran and all subjects signed informed consent.
4. Results
The 20 patients including 6 females (70%) and 14 males (30%) aged 15 to 87 years old (mean age of 53.2 years) were enrolled and completed the study.
There were no differences between 2 groups in age, gender, being edentulous, type of tumor radiation dose and weight. (P > 0.05) weight loss can be a good marker of their malnutrition status.
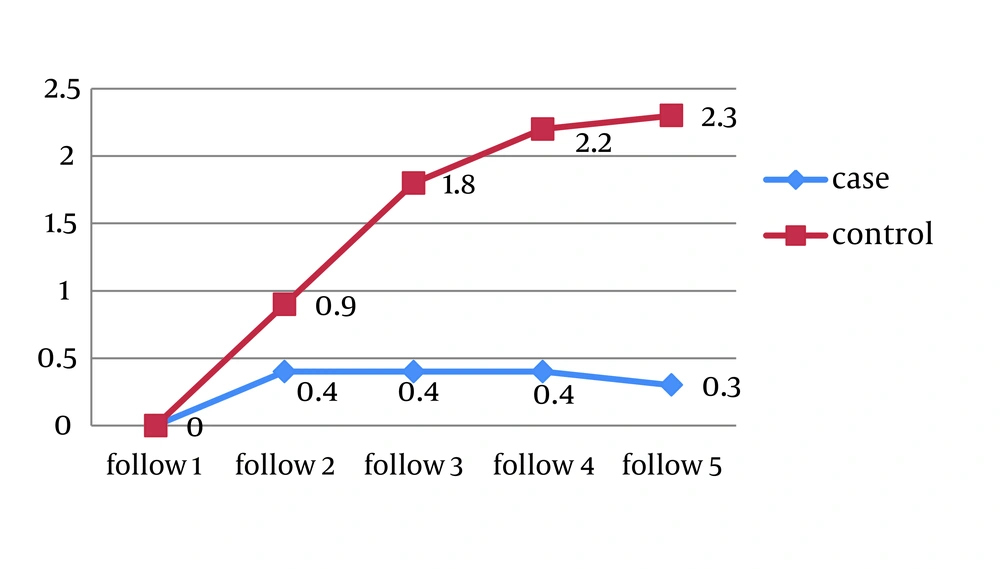
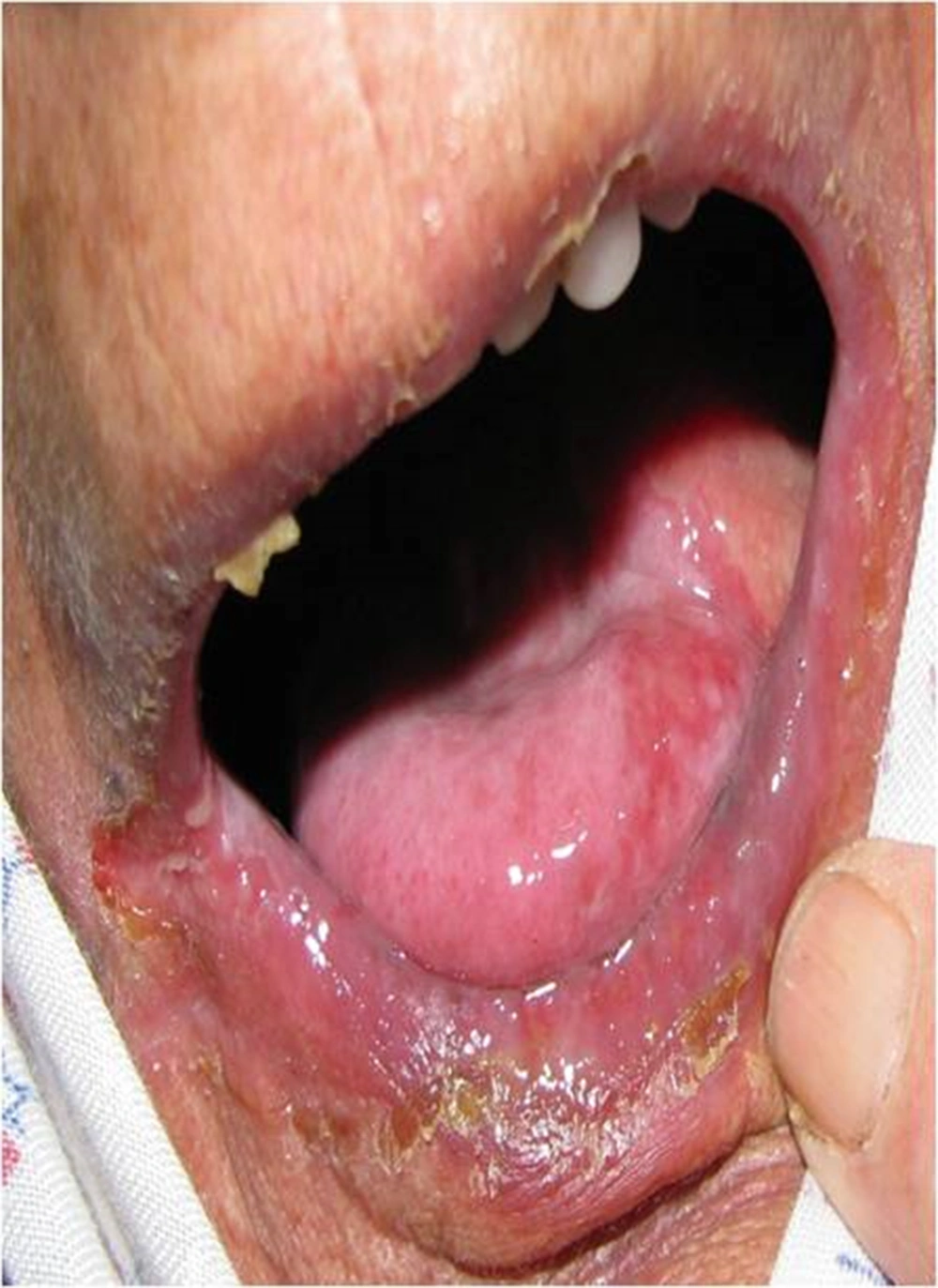
The mucositis score of NCI-CTC at the end of each week in the propolis group was significantly lower than placebo group (Figure 1).
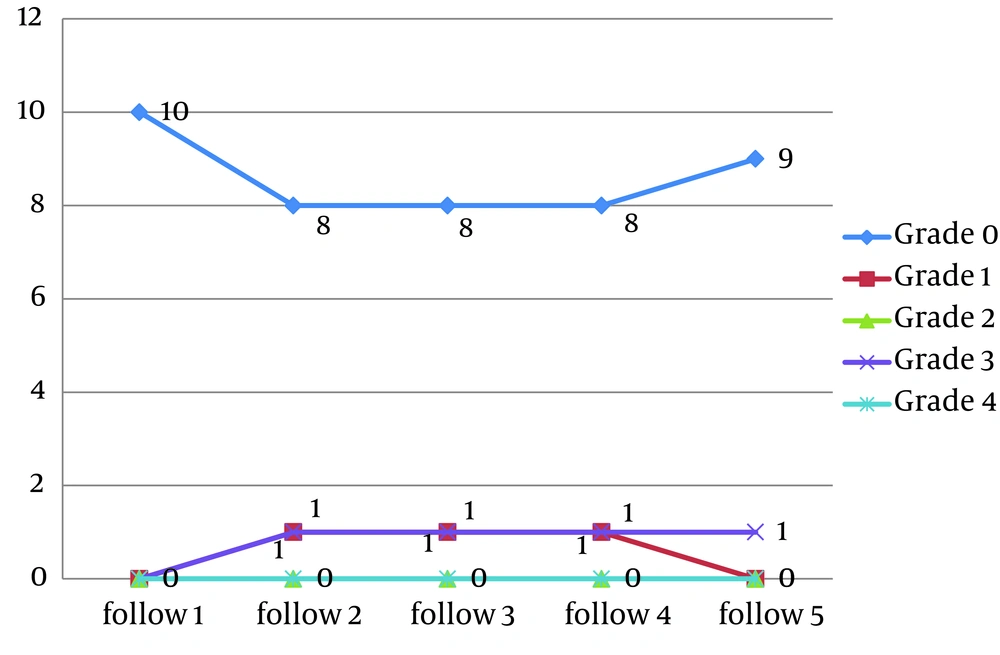
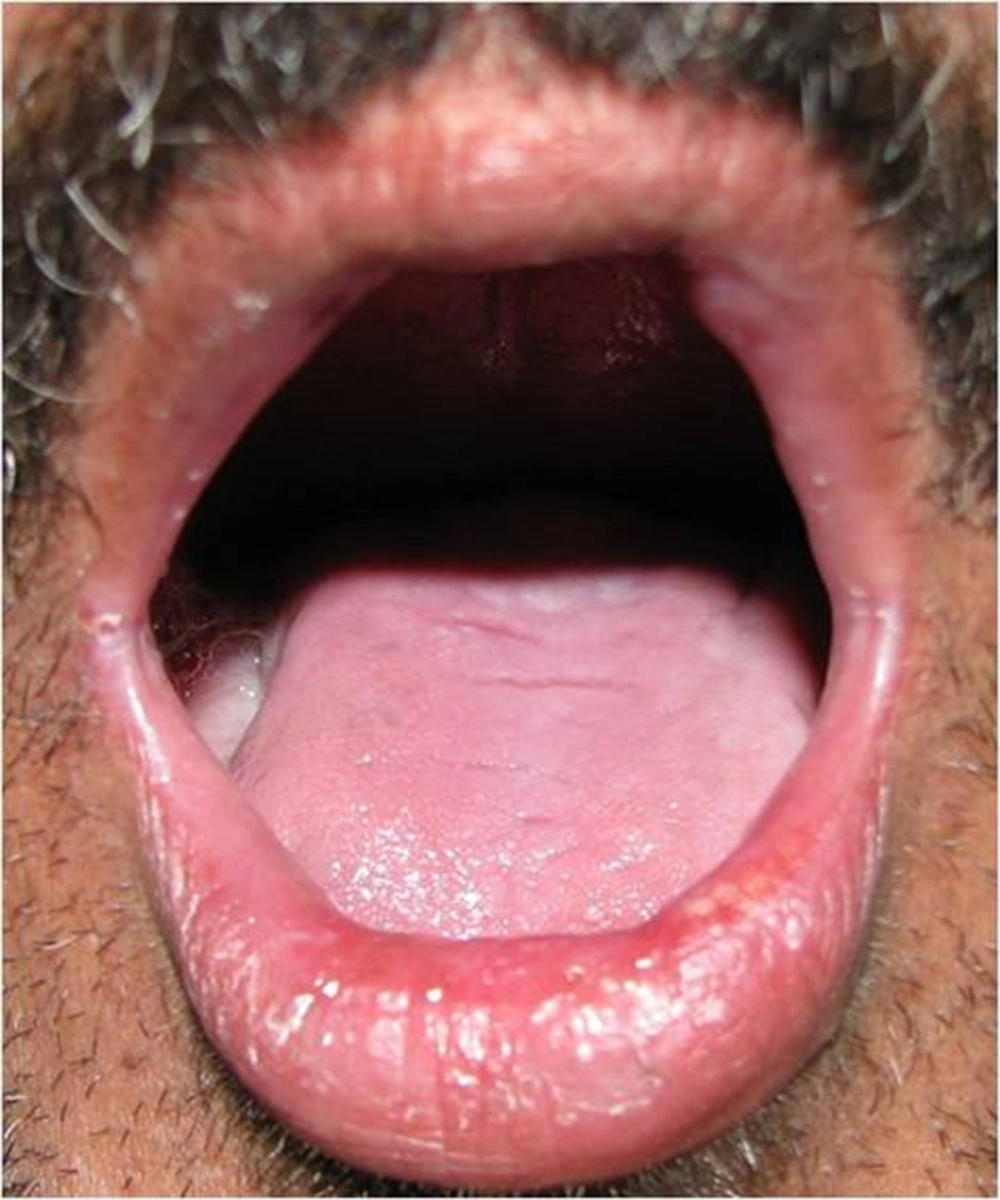
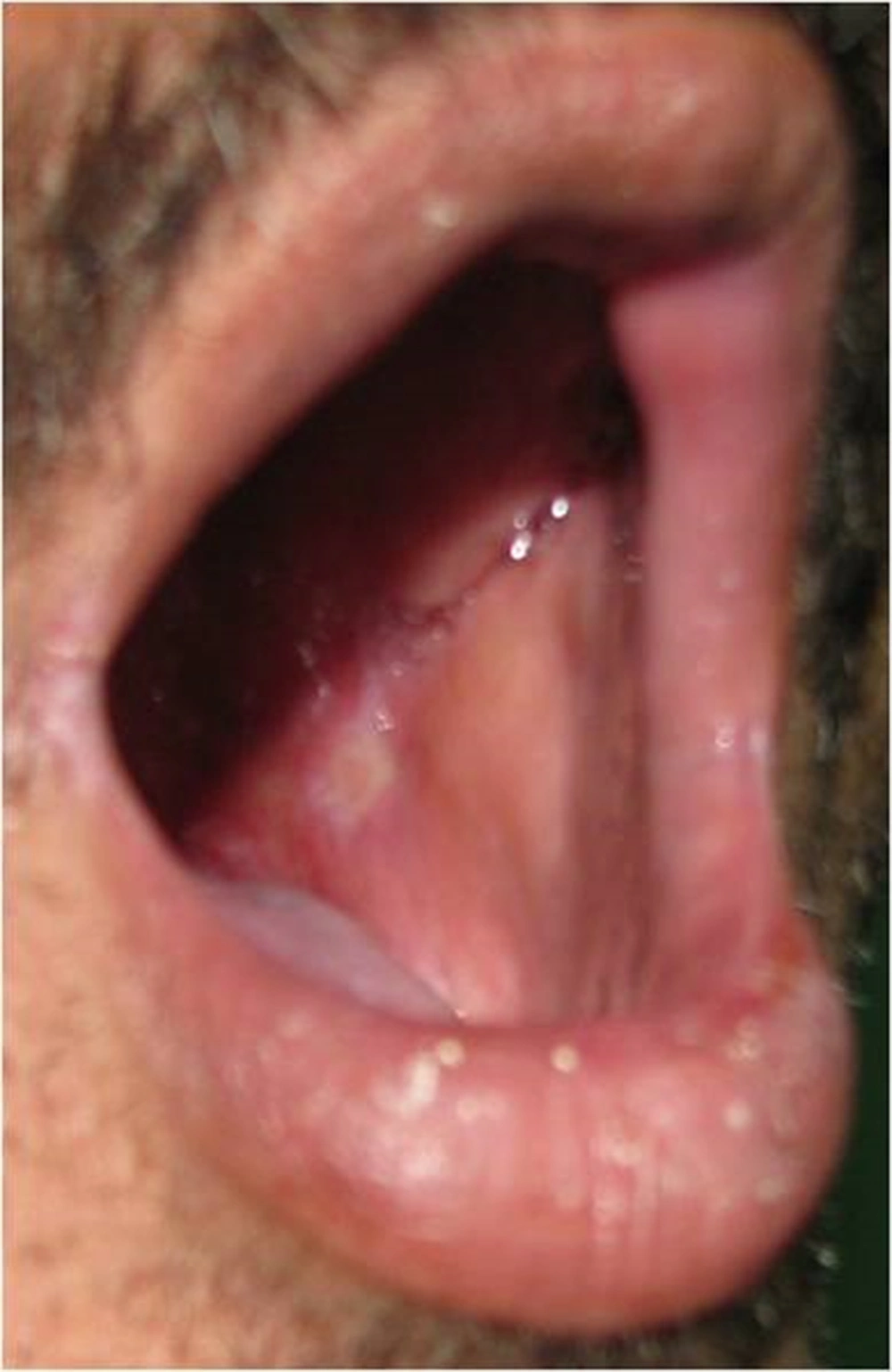
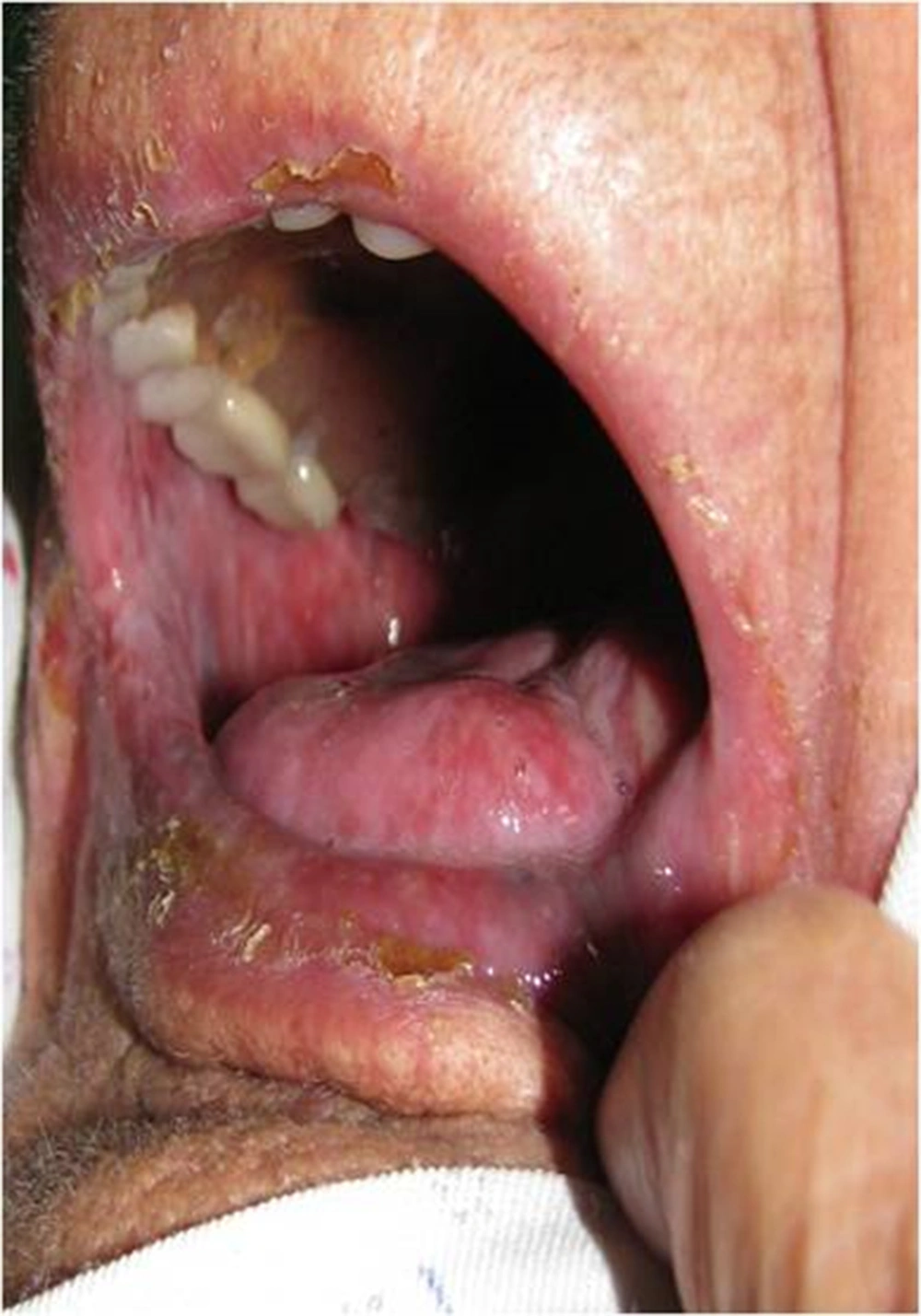
In propolis group 8 patients showed no evidence of mucositis during the radiotherapy course. (Figures 2-6).
The mean rank of NCI-CTC at the end of each week in two groups was estimated (Table 1).
| Group | Mean Rank | Number |
|---|---|---|
| Case | 2.65 | 10 |
| Control | 1.30 | 10 |
| Case | 3.15 | 10 |
| Control | 2.35 | 10 |
| Case | 3.15 | 10 |
| Control | 3.45 | 10 |
| Case | 3.15 | 10 |
| Control | 3.90 | 10 |
| Case | 2.90 | 10 |
| Control | 4.00 | 10 |
Mean Rank and Number of Both Groups
Weight loss was measured in both groups. The mean weight loss in the case group was 200 g (9 patients showed no weight loss), while in the control group the mean weight loss was 3.4 kg, and the difference was significant (P = 0.029).
5. Discussion
Benderli Cihan and Deniz in 2011 reported propolis as an effective drug in reducing the severity of radiation induced oral mucositis in rats (19). Ghassemi and Zabihi also confirmed the effect of ethanolic extract of propolis on radiation induced mucositis in rats (20).
To authors’ knowledge, there is no available human study report about the effects of propolis on mucositis until this paper was published. This study showed encouraging results for the prevention and treatment of radiation induced mucositis.
The effective dose of propolis suggested in two mentioned animal study was 100 mg/kg/day (19, 20). In this study we used polyphenol 1.4 mg/kg/day which is equal to 70 mg propolis per day. We provide mouth wash containing this exact daily dose in a swish and swallow way to benefit from both topical and systemic effects (21).
Propolis has a broad biological effect that help preventing and treating mucositis. It is antifungal, antibacterial and antiviral agent, besides healing, anti-ulceration, anti-inflammatory, antioxidant effects which are due to rich flavenoid agents in propolis (19). The most important effect of flavenoids is oxygen free radical scavenging and several studies have confirmed the role of deactivation of free radical (21).
Some researchers proved the hemathopoetic recovery effects of propolis derived compounds. The results of this research confirmed this hypothesis on preventing and therapeutic effects of oral mucositis, but the greatest limitation at this study was small sample size, and we assessed just one exact concentration in mouthwash (22).
While assessing the radioprotective effect of quercetin and the ethanolic extract of propolis, (EEP) Benković showed that the alkaline comet assay proved that both natural compounds, especially when given as pre-treatment, protect against primary leukocyte DNA damage in mice. In the tested concentrations, EEP and quercetin were not genotoxic to non-irradiated mice. AET, however, resulted in a slight, but not significant increase in DNA damage. Although the results of this study show the radioprotective potential of the test compounds, further investigation is needed to clarify the underlying protection mechanisms (23).
Propolis is a product with rich flavenoid that could be suggested as a preventing and economic treatment agent in the medical approach of radiation induced oral mucositis. Different ways and doses at propolis on larger size sample were suggested for future studies.
In conclusion this study suggests that propolis water based mouthwash is safe and effective in the prevention and treatment for radiotherapy induced mucositis. Doing more studies with wider scope of samples different consentration of propolis from all over the world is highly recommended.