1. Background
Breast cancer is the most common malignant disease in women and is the second leading cause of death globally. Despite many advances in early diagnosis and treatment, there are about 50,000 cases of metastases were reported per year. According to the World Health Organization (WHO), one in every 8 to 10 women suffers from breast cancer which accounts about 19% of cancer-related mortality and 22% of all women’s cancers in Iran (1).
The HER2 and MUC1 proteins are considered as the most important diagnostic and therapeutic targets for the treatment of breast cancer. MUC1 is one of the most important proteins on the epithelial cells of breast tissue (2). In tumor tissues, the polarity of MUC1 distribution in epithelial cells is eliminated, and secreted into biological fluids which can be measured by immunological assays (3). In more than 30% of breast cancers over the expression of the HER2/neu gene has been observed (4). HER2 is a growth factor receptor produced by erbB-2 gene and has tyrosine kinase activity in the cells (5). Regarding the critical role of HER2 and MUC1 in the diagnosis of breast cancer, choosing the appropriate method for determining their expression levels in cancer cells is very important (1, 6).
Based on the reports, stimulating the immune system using HER2 and MUC1 proteins can lead to inhibitory effects on the development of breast cancer tumors in mice (6, 7). Oral immunization is considered as a preferred route for delivery of the vaccine, given the possibility of inducing both mucosal and systemic immunity (8). On the other hand, one of the important factors for enhancing the efficacy of a vaccine is the choice of adjuvant. Chitosan and its derivatives have been widely studied for oral delivery of drug proteins and antigens, because of mucosal properties and high absorption (9). Oral vaccines are not adequately absorbed after administration and they required an agent for enhancing immunological response such as adjuvants or encapsulation in liposomes. Due to the instability and low absorption efficiency of liposomes, the use of polymeric nanoparticles has been considered as an alternative method (10). Other advantages of nanoparticles are including slow release and intracellular absorption pathways in the digestive tract (11). According to the previous study, the chimeric HER2-MUC1 (HM) protein can be considered as a vaccine candidate for breast cancer (7).
2. Objectives
The main objective of the current study is to produce chitosan nanoparticles containing HM protein and evaluation of its immunological response in the animal model.
3. Methods
3.1. Bacterial Strains, Plasmids, and HER2-MUC1 Construct
The pET-28a plasmid harboring the HER2-MUC1 was available from the previous study (12). The HER2-MUC1 chimeric gene composed of the antigenic extracellular domain of HER2 (480 - 620 aa) and MUC1 (220 - 360 aa) which attached together by a hydrophilic linker (12). Extraction of the pET-28a plasmid harboring the HER2-MUC1 gene was performed by the mini-preparation method using the GenetBio kit. To confirm the recombinant construct, the PCR was performed using universal T7 primers.
3.2. Protein Expression of the HER2-MUC1
In order to evaluation of HER2-MUC1 expression, five recombinant colonies of the BL21DE3 containing the recombinant plasmid were grown at 37°C (LB broth containing 50 μg/mL kanamycin). After OD600 reaches 0.6, IPTG was added to the culture medium at a final concentration of 1 mM and incubated for 5 hours at 37°C.
3.3. Protein Solubility
For analysis of protein solubility, expression was performed in large-scale 50 mL culture and bacterial precipitate dissolved in 5 mL of native buffer (50 mM NaH2PO4 and 300 mM NaCl, pH 8) and mix thoroughly. In addition, lysozyme (1 mg/mL) and sonication (6 × 10 s with 10 seconds pauses at 200 - 300 W) were applied to ensure complete cell lysis. After centrifugation at 13000 g for 10 minutes, supernatant containing soluble protein was collected and the pellet resuspended in a denaturing buffer (100 mM NaH2PO4, 10 mM Tris-Cl and 8 m urea, pH = 8) and incubated in 37°C for 1 hour and the samples were analyzed on 12% SDS-PAGE gel.
3.4. Purification of the Recombinant HER2-MUC1
After sonication, the lysate was centrifuged (15 minutes, 10000 g, 4°C) and the supernatant was applied on a Nickel-nitrilotriacetic acid (Ni-NTA) affinity chromatography column (Qiagen) and the purification steps were performed according to the manufacturer instructions. The column was equilibrated with lysis buffer and the protein solution was loaded onto the column at a flow rate of 0.5 mL/min. The impurity was removed two times by washing the column with washing buffer (100 mM NaH2PO4, 10 mM Tris-Cl, 8 M urea pH = 5.9). The protein was eluted with elution buffer (100 mM NaH2PO4, 10 mM Tris-Cl, 8 M urea) at pH = 4.5. Protein concentration was determined by the Bradford method with BSA (bovine serum albumin) as a standard (13).
3.5. Western Blotting to Confirm the Expressed Protein
The recombinant protein was separated by 12% SDS-PAGE and electrotransferred onto PVDF membrane (Roche). The membrane was blocked with 5% non-fat skim milk in TBS buffer (50 mM Tris-Cl, 150 mM NaCl, pH = 7.5) containing 0.05% Tween 20 (37°C, 2 hours). The membrane was incubated with HRP-conjugated mouse anti-poly His-tag antibody (1:2000 Roche). Finally, the membrane was soaked in 3, 3’-Diaminobenzidine tablet (DAB Reagents; Sigma) for signal development (13).
3.6. Preparation of Chitosan Nanoparticle
The purified protein (rHer2-Muc1) was added dropwise to 7.5 mL of chitosan solution (2 mg/mL in 2% acetic acid). The pH of the solution was adjusted to 5.5 using NaOH and placed on a stirrer for 30 minutes until it was thoroughly mixed. In the next step, 5 mL of TPP was added gradually to the solution containing chitosan and purified protein. Sonication was performed (3 times for 20 seconds with high power) to prevent the accumulation of nanoparticles. A solution containing BSA (1 mg/mL) was prepared as negative control under the same conditions. Finally, the solutions were centrifuged at 13000 rpm for 45 minutes at 4°C (14).
3.7. Determine the Loading Percentage and Size of the Nanoparticles
After centrifugation, 100 μL of supernatant was separated and the concentration of the recombinant protein measured. The percentage of loaded protein on the nanoparticle was calculated. Moreover, Bradford test was used for determining the release rate of the rHER2-MUC1 from nanoparticles. The size of the nanoparticles was measured by a particle size analyzer, known as the Zeta Sizer.
3.8. Mice Immunization
To determine the antigenicity of the recombinant Her2-Muc1, 12 BALB/c mice (female, 6 - 7 weeks old, Pasteur Institute, Tehran, Iran) were randomly divided into four groups. Group I was injected subcutaneously with 100 µg of nanoparticle Her2-Muc1. The group II and III were immunized via an oral and oral-injection route with 100 µg of nanoparticle Her2-Muc1, respectively. Group IV was immunized with PBS as the negative control. Serum was prepared from the blood sample of each mice group (blood was transferred to vials and allowed to clot for 30 minutes, then serum was collected by centrifugation) and frozen at -70°C until use. The serum samples of each mice group were prepared and then pooled for immunological analyses.
3.9. Measurement of IgG and IgA by ELISA
The collected sera were subjected to ELISA-based antibody titer assays. The purified r HER2-MUC1 (500 ng/well) were used to load Maxisorb plates (Nunc, Denmark) with 100 µL bicarbonate buffer (15 mM Na2CO3 and 35 mM NaHCO3) incubated 2 hours at 37°C. The wells were blocked for 2 hours at 37°C by the addition of 200 µL of 3% (w/v) non-fat skim milk in PBST (PBS containing 0.05% Tween-20) and washed three times with PBST. The wells incubated with serially diluted serum from immunized mice in triplicate at 100 µL/well for 1 hour at 37°C. The bound antibodies were detected with HRP-conjugated goat anti-mouse IgG (Sigma) and HRP-conjugated rabbit anti-mouse IgA (Sigma) in a 1:5000 dilution for 1 hour and washed three times with PBST. The reaction was developed with O-phenylenediamine (OPD) as a substrate (Sigma) for 15 minutes at room temperature in the dark. Sulfuric acid (2.5 M) was used to stop the reaction and the absorbance score was measured at 492 nm in an ELISA reader.
3.10. Evaluation of Proliferation by MTT
Cell proliferation was tested using a 3-(4, 5-dimethylthiazol-2-yl)- 2, 5-diphenyl tetrazolium bromide (MTT) assay. For the assay, lymphocytes were freshly isolated from spleen and plated in 96-well flat bottom tissue culture plate at a concentration of 1 × 105 cells/well. At 72 hours of culture, 10 µL of MTT solution was added per well. After 3 hours of incubation, colored crystals of formazan were dissolved with a 100 µL of dissolving solution. Plates were kept on the shaker for 5 minutes and optical density (O.D.) was read on ELISA reader at 540 nm.
4. Results
4.1. Confirmation of pET-28a Plasmid Containing HER2-MUC1 Gene
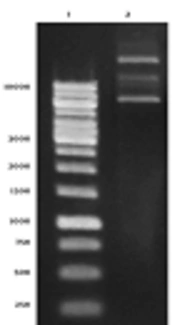
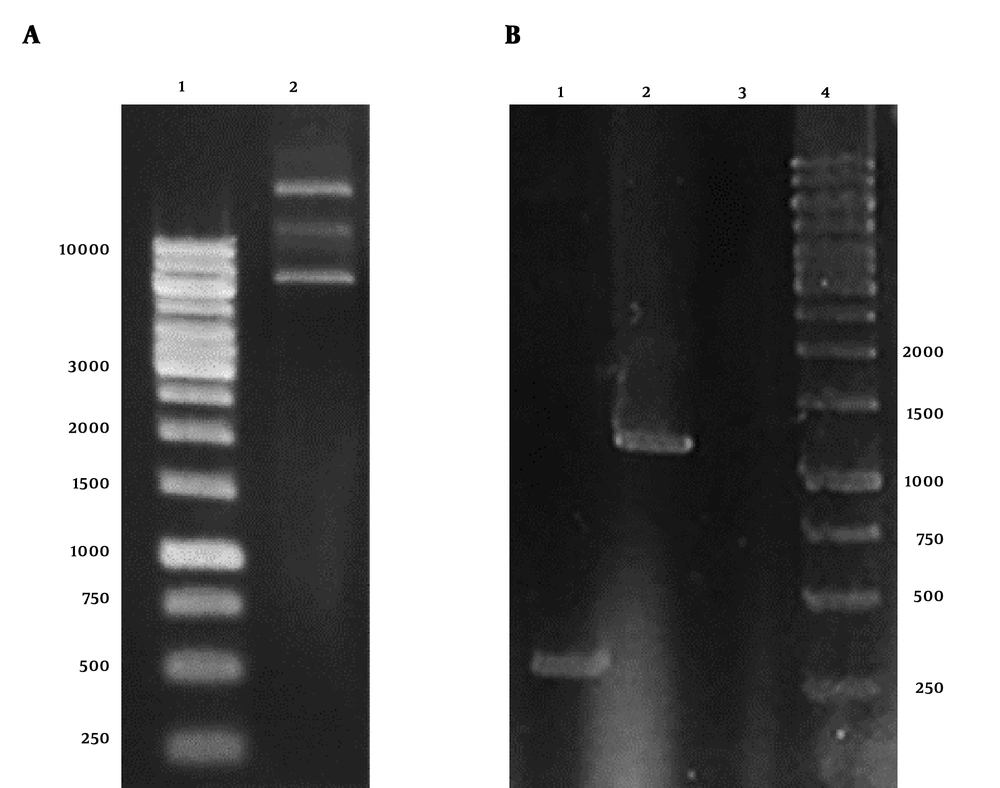
The result of plasmid extraction was illustrated in Figure 1A. To ensure the presence of stx2b gene in pET28a plasmid, PCR was performed with T7 universal primers. The HER2-MUC1 fragment 1250 bp was observed on 1% agarose gel (Figure 1B).
Electrophoresis of pET28a-HER2-MUC1 plasmid and PCR product on 1% agarose gel. A, Extracted plasmid 1, DNA marker; 2, the plasmid pET28a containing HER2-MUC1 gene. B, PCR product with T7 primers; 1, PCR product with pET28a; 2, PCR product with pET28a-her2-muc1; 3, negative control; 4, DNA marker.
4.2. Expression of Recombinant HER2-MUC1 Gene
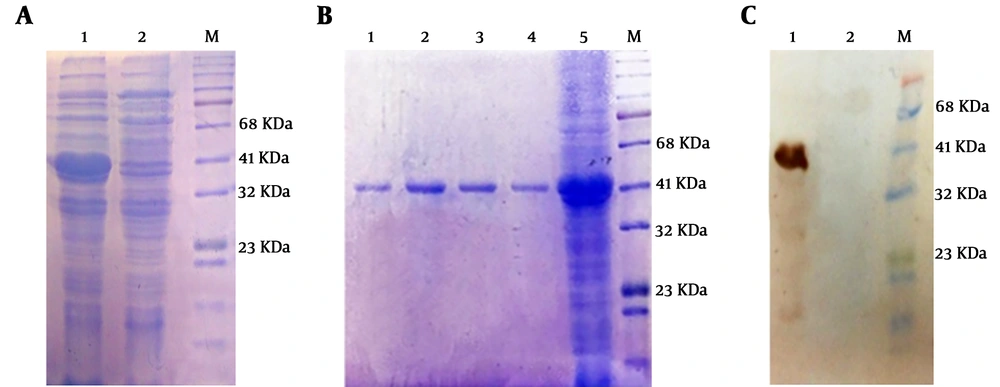
Expression of recombinant protein was analyzed on 12% SDS-PAGE and desired HER2-MUC1 (40 kDa) in fusion with 6x His-tag (C-terminal) were detected. The recombinant protein produced as inclusion bodies (IB) which subsequently solubilized using 8 M urea (Figure 2A). Purification of the recombinant protein was performed by Ni-NTA affinity chromatography under denaturing condition (Figure 2B). Estimation of the purified recombinant protein by the Bradford method was indicated that the concentration of the HER2-MUC1 was 356 μg/mL which used for the preparation of nanoparticles.
Expression, purification, and western blotting of recombinant HER2-MUC1 protein. A, Electrophoresis of recombinant HER2-MUC1 protein expression on 12% SDS-PAGE M, protein ladder; 1, inclusion body in the extraction buffer containing urea; 2, lysate of bacteria in the native buffer. B, Electrophoresis of the recombinant protein purified by the Ni-NTA column on 12% SDS-PAGE. 1 - 4, purified protein (Elution Buffer); 5, bacterial lysate; M, protein ladder. C, Western blotting with the anti-His antibody. 1, recombinant HER2-MUC1 protein; 2, negative control; M, protein ladder.
4.3. Western Blotting to Confirm the Expressed Protein of HER2-MUC1
The authenticity of recombinant HER2-MUC1 (40 kDa) proteins was confirmed by anti-poly His-tag antibody. In contrast, no reactivity was observed in the negative control (Figure 2C).
4.4. Determining the Nanoparticle Size Containing HER2-MUC1 Protein
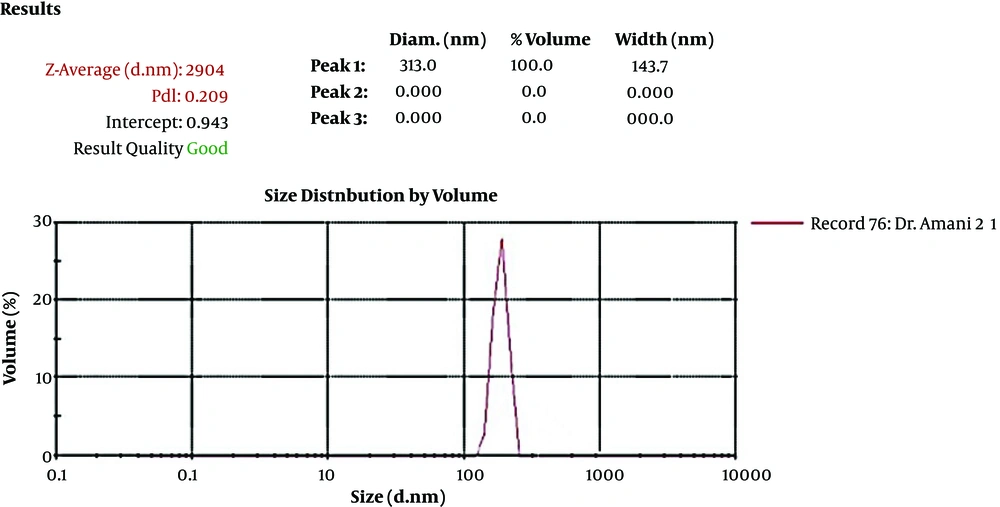
An antigen loading on the nanoparticle showed that 88% of the recombinant HER2-MUC1 protein was nanocapsules. The size, uniformity, and distribution of chitosan nanoparticles were determined by the DLS (Malvern) analyzer (Figure 3).
4.5. Evaluation of IgG and IgA Antibodies by ELISA
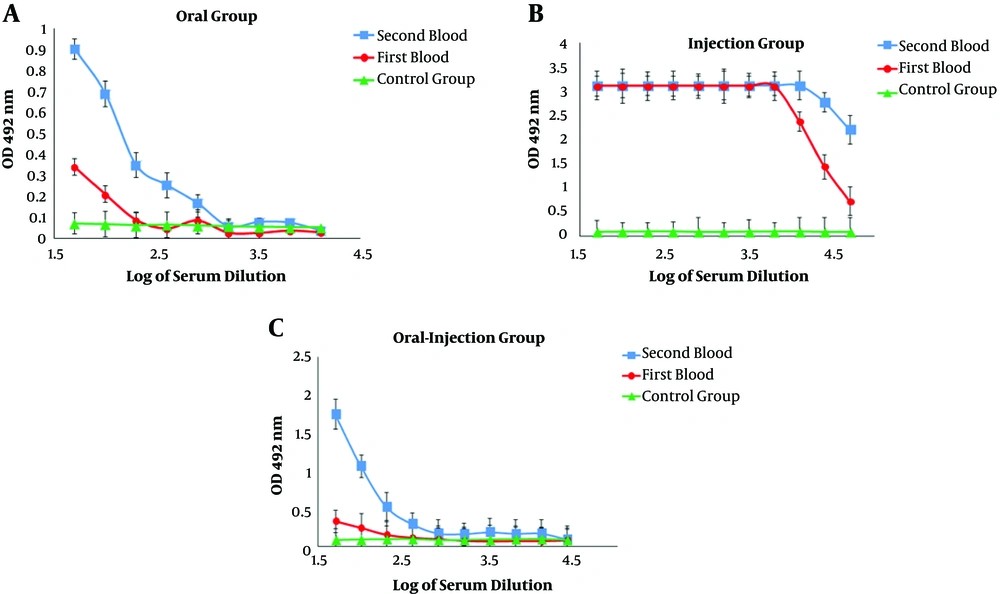
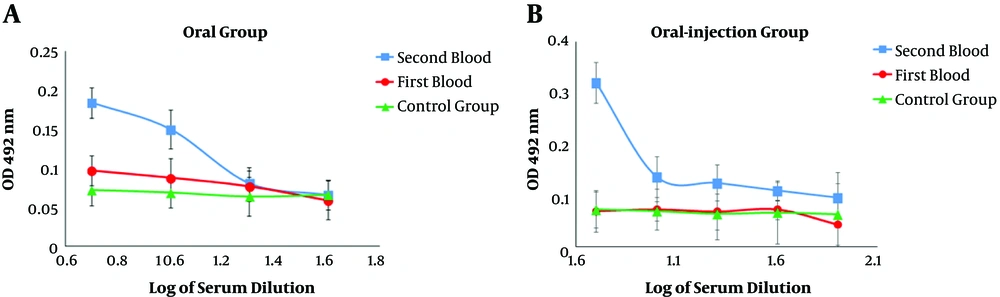
The results of serum ELISA in immunized mice indicated a systemic response induction and increased serum IgG titer against the recombinant HER2-MUC1 protein. In addition, in the second administration, the serum IgG level in the oral-injection group was increased compared to the first administration and there was the significant difference (P < 0.05) in antibody titration (Figure 4A - C). In order to measure the amount of secreted IgA, serum and fecal IgA levels were evaluated in the samples after oral and oral-injection administration. In the oral and oral-injection group, a significant increase was observed after the final booster (Figure 5A and B).
4.6. MTT Assay
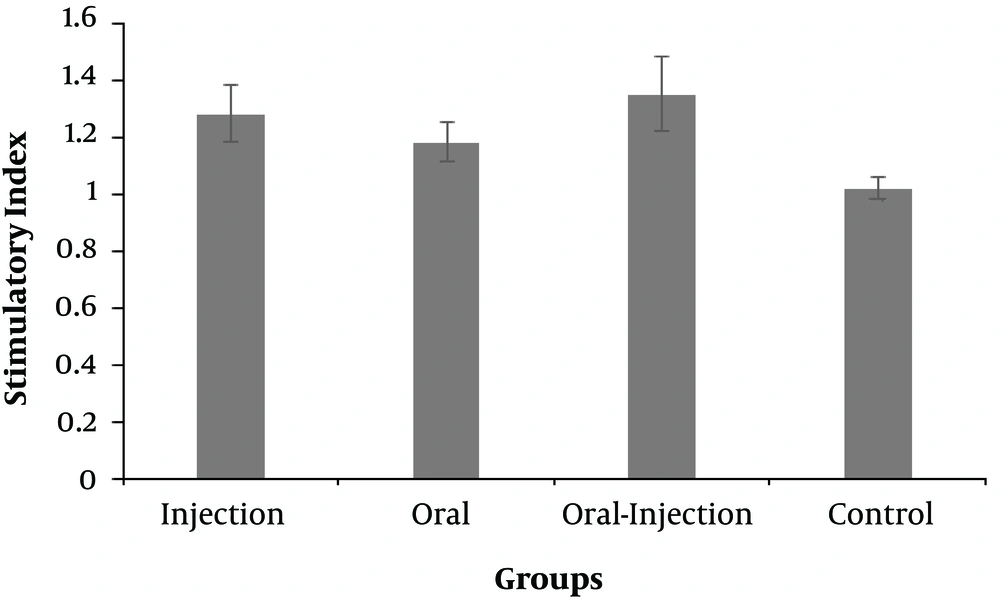
The response of lymphocytes to nanoparticles containing recombinant HER2-MUC1 protein as the stimulatory index was determined (Figure 6). The group of oral-injection mice had the highest titers compared to the control group, followed by an injection and oral group.
5. Discussion
Conventional methods for the treatment and control of breast cancer are the use of anti-tumor drugs, radiotherapy, and surgery. Regarding the use of chemical drugs, extensive resistance has been observed, and after metastasis, surgery and radiation therapy will not be beneficial (15). For this reason, new strategies such as the early detection of breast cancer have been suggested with the help of tumor-dependent antigens. With this regard, the use of Herceptin is very beneficial for patients who have diagnosed as HER2 positive (15, 16). HER2 is a mutated analog receptor for EGF (epithelial growth factor receptor) and involved in the regulation and development of the natural gland of the breast. Increasing the expression of wild-type HER2 affected tumorigenesis. The active HER2 protein causes uncontrolled proliferation of cancer cells and disrupts the normal organization of epithelial cells. Breast cancer patients with HER2-positive tumor cells in the bone marrow are subjected to higher risk for recurrence of the next metastasis, as compared to patients with a release of tumor cells lacking HER2 expression (17).
The MUC1 protein also can act as an antigen for stimulating immune response due to incomplete glycosylation. It has revealed that MUC1 is less or not glycosylated in the cancer cells (18). Although the main function of MUC1 is not well known, it probably involved in cellular attachment and reduced cell-to-cell interactions due to disruption of E Cadherin function in cellular adhesion (19). Based on the in vitro studies, the overexpression of MUC1 could increase the cellular invasion (20, 21). Moreover, autoantibodies against MUC1 and HER2 can be detected in both malignant and healthy cells. It has been revealed that the detection of anti-MUC1 and anti-HER2 antibodies could be used as a good diagnostic factor in the early stages of disease (18, 20). In the previous study, recombinant HER2-MUC1 protein was expressed in E. coli as an appropriate vaccine candidate against breast cancer. The HM chimeric protein consists of the extracellular domain of MUC1 and HER2 which are located on the cell surface with minimum post-translational modifications (7, 12). In the present study, the production of chitosan nanoparticle containing HM protein and its immunization in a BALB/c mice was evaluated.
The amplification of the target gene by PCR confirms the correct cloning of the gene in pET28a vector. The immunoblotting result indicated that the presence of a 40 kDa band, which is equivalent to the predicted molecular weight for the HM chimeric protein. Regarding the fact that the His-tag was designed at the carboxyl end of the recombinant protein, it can be expected that the protein synthesis machine translated a mature protein from mRNA.
The production of nanoparticles containing purified recombinant protein using chitosan was successfully performed and the results indicated that the nanoparticle size and the loading efficiency were 205.2 nm and 70%, respectively. The physicochemical properties of nanoparticles, such as type and size of the particle, the percentage of loading, and efficiency of protein release are the factors that influence the immunological response (14, 22). In another study, which used chitosan nanoparticles to design a vaccine candidate against Newcastle disease, the size of the nanoparticles was 699nm and the loading efficiency was 98% (23, 24). Another study found that the chimeric protein, which was encapsulated in PLGA has 25.27 nm in size and 91.96% in the loading efficiency (25). Furthermore, the best size of the nanoparticle was obtained at a concentration of 1 mg/mL chitosan. In the present study, the produced nanoparticle had a good size but the loading efficiency was relatively low (about 75%), which can be associated with the factors such as the nature of the nanoparticle, the molecular weight, and the physicochemical properties of the antigen (14, 22). Immunization evaluation indicated that the chimeric HM protein has the good potential to stimulate mucosal and humoral immune response. The results of ELISA showed a potent immune response in the injection group and the high level of IgG production. IgG titration in oral and oral-injection groups revealed that oral administration of this nanoparticle stimulated both mucosal and humoral immune system. Furthermore, higher IgG levels were detected in the injection group compared to the other groups, which was consistent with other reports (22). The comparison of IgG and IgA titration showed that the rate of stimulation of the humoral and mucosal immune system in the oral and oral-injection group was higher than the injection group, which were in line with previous findings (15). The spleen cells of immunized mice analysis showed that the oral-injection group had the highest immune response and cellular immunity in the oral-injection group was induced as well as mucosal and humoral immunity.
In the previous study, stimulation of the immune system was further directed towards humoral immunity, which was not desirable (7). The purpose of this study was to stimulate the humoral and cellular immune system simultaneously. Therefore, the nanoparticles of the HM protein could effectively stimulate the immune system via the oral-injection route. In addition, to the production of IgG and IgA, the cellular immunity was also stimulated and the proliferation of T-cells in the spleen could be detected.
5.1. Conclusion
It is suggested that the chitosan nanoparticles containing HER2-MUC1 protein can be applied as a vaccine candidate against breast cancer.