1. Context
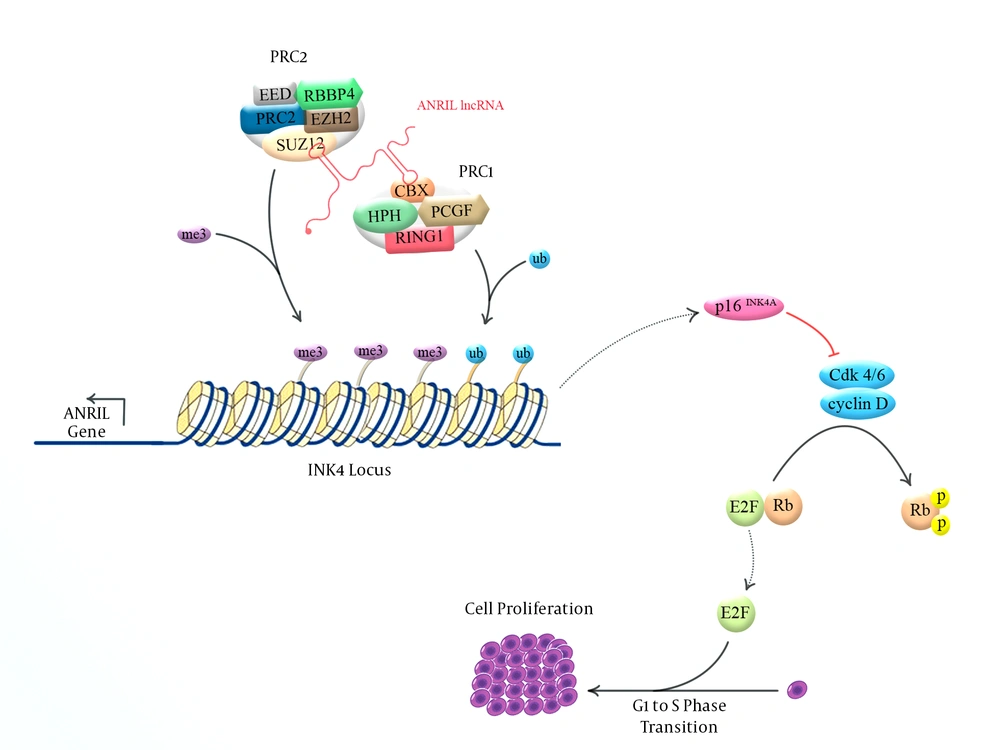
Long non-coding RNAs (lncRNAs) are a group of transcripts with sizes greater than 200 nucleotides that instead of being translated into protein, themselves exert crucial functional roles (1). Almost any fundamental aspect of cell physiology is a subject of regulation by lncRNAs (2-5). Their participation in a wide range of cancers has also been evaluated (6-11). ANRIL (antisense non-coding RNA in the INK4 locus) is one of the oncogenic lncRNAs, which is located at the 9p21.3 locus (12). This locus has been recognized as a common genetic susceptibility locus for coronary disease, intracranial aneurysm, type 2 diabetes, and several malignancies through genome-wide association studies (GWAS) (13). The 9p21.3 locus encompasses p15/CDKN2B-p16/CDKN2A-p14/ARF gene cluster as well. ANRIL has been shown to regulate the expression of this gene cluster through recruitment of polycomb repressive complexes PRC2 and PRC1 to this locus (14) (Figure 1). ANRIL-mediated recruitment of these complexes alters the epigenetic chromatin shape and, hence, suppresses the expression of this gene cluster in a cis-acting manner (15). PRC1 and PRC2 participate in chromatin remodeling, suppress transcription of several genes and interact with key signaling pathways. Their regulatory role on stem cell pluripotency provides further evidences for their participation in cancer pathogenesis. PRC2 and PRC1 suppressive regulatory function is exerted through two diverse histone modifications, trimethylation of lysine 27 on histone H3 (H3K27me3) and monoubiquitination of histone H2A (H2AK119ub) (16). ANRIL-mediated silencing of the CDKN2B/CDKN2A/ARF locus is exerted though ANRIL interaction with EZH2 (PRC2) and CBX7 (PRC1) (17). The observed up-regulation of ANRIL and CBX7 in prostate cancer tissues and the ability of ANRIL to bind with CBX7 provided further evidences for contribution of ANRIL in suppression of the CDKN2A/B locus (14). Moreover, EZH2 is a histone methyltransferase, which participates in silencing of p15/CDKN2B-p16/CDKN2A-p14/ARF cluster by tri-methylating H3K27 in the corresponding promoters (18). The p15/CDKN2B-p16/CDKN2A-p14/ARF-ANRIL genes contribute in the regulation of senescence and apoptosis. Alterations in tumor suppressor genes encoded by p15/CDKN2B-p16/CDKN2A-p14/ARF locus have been associated with a wide range of malignancies (19-21), implying the parallel role of ANRIL in the pathogenesis of these malignancies. Consequently, several studies have focused on the evaluation of ANRIL expression or genomic variations in human cancers.
2. Evidence Acquisition
We searched the PubMed, Google Scholar, EMBASE, and Web of Science databases till February 2018 with the key words "ANRIL" OR "antisense non-coding RNA in the INK4 locus" AND "cancer" OR "tumor" OR "malignancy". We only included original articles written in English, described the mechanism of ANRIL participation in malignancies and provided enough patients' data. We excluded other types of papers from the study.
3. Results
Several studies have pointed the significance of ANRIL in diverse human malignancies. Table 1 shows the summary of these studies.
| Cancer Type | Expression | Role | Target Genes | Variant Associated with Cancer Risk | Variant Associated with Therapeutic Response | References |
|---|---|---|---|---|---|---|
| Lung cancer | Up | Associated with TNM stage, cisplatin resistance and poor prognosis | c-Myc, KLF2, P21 | - | rs1333049 (associated with severe overall toxicity of cisplatin), rs10120688 and rs1333049 (associated with response to platinum) | (22-25) |
| Hepatocellular carcinoma | Up | Promotes cell proliferation | miR-122-5p, KLF2 | - | - | (26, 27) |
| Prostate cancer | Up | Promotes cell proliferation and migration | CBX7 | rs4977574, rs1333048, rs10757278 | - | (14, 28, 29) |
| Breast cancer | Up | Promotes cell proliferation | p15/CDKN2B-p16/CDKN2A-p14/ARF gene cluster | An rs1333045, rs4977574, rs1333048 and rs10757278 haplotype, rs11515 | - | (12, 53, 30) |
| Gastric cancer | Up | Promotes cell proliferation, Associated with cisplatin- and 5-fluorouracil (5-FU)-resistance | MDR1, MRP1, miR-449a, p15Ink4B, p16Ink4A | - | - | (31, 32) |
| Colorectal cancer | Up | Promotes cell proliferation, migration and invasion | - | - | - | (33) |
| Cervical cancer | Up | Promotes cell proliferation | miR-186, PI3K/Akt pathway, p15, p16 | - | - | (34) |
| Ovarian cancer | Up | Promotes cell proliferation | P15INK4, Bcl-2 | - | - | (35) |
| Oral squamous cell carcinoma | Up | Promotes cell proliferation, inhibits metastasis, associated with cisplatin- resistance | - | - | - | |
| Esophageal squamous cell carcinoma (ESCC) | Up | Promotes cell proliferation | p15INK4b, transforming growth factor β1 (TGFβ1) | - | - | (36) |
| Nasopharyngeal carcinoma (NPC) | Up | Promotes cell proliferation, decreases apoptosis, increases radioresistance, reprograms cell glucose metabolism and induces side-population stem-like cancer cells | miR-125a, miR-let- | - | - | (37, 38) |
| Acute lymphoblastic leukemia (ALL) | - | - | - | - | rs564398 correlated with ALL phenotype | (39) |
| Multiple myeloma (MM) | - | Associated with melphalan-mediated apoptosis | p14ARF, p53 | - | rs2151280 associated with worse progression-free survival | (40) |
3.1. ANRIL in Breast Cancer
In a large cohort of patients with breast cancer (n = 456), ANRIL over-expression has been detected in 19.7% of invasive breast carcinomas, particularly in the aggressive triple negative breast cancer (TNBC) subtype (16). Moreover, its expression level was correlated with estrogen receptor (ER) and progesterone receptor (PR) status but no patients' survival. A strong positive correlation has been detected between mRNA expression levels of the ANRIL, ARF, p15, and p16 pointing to the presence of a complicated network between these genes (16). In spite of the proposed role for ANRIL in epithelial-mesenchymal transition (EMT), no significant associations were found between ANRIL and EMT-associated markers such as TWIST1, ZEB1, ZEB2, SNAI1/Snail, SNAI2/Slug, VIM/Vimentin, CDH2/N-cadherin, CDH1/E-cadherin, and ZO-1 (16).
More recently, ANRIL has been shown to be over-expressed in TNBC tumor tissue and cell lines compared to noncancerous tissue and non-TNBC cells. Such elevated expression was accompanied by poor prognosis. In vivo functional studies confirmed the role of ANRIL as molecular 'sponge' for miR-199a in TNBC (30).
The rs11515 SNP located in the 5' region of ANRIL has been shown to be associated with breast cancer risk in a sample of 200 patients and 200 healthy subjects from New Zealand. Notably, the CG genotype of this SNP has been correlated with increased ANRIL and decreased p16INK4a expression levels suggestive of the decreased expression of p16INK4a as a putative mechanism for contribution of rs11515 CG genotype in cancer risk (41). Recently, we genotyped rs1333045, rs4977574, rs1333048, and rs10757278 SNPs in 122 patients with breast cancer and 200 normal age-matched subjects and found TCGA haplotype to be associated with breast cancer risk in Iranian population (12).
3.2. ANRIL in Prostate Cancer
The elevated expression of ANRIL as well as chromobox 7 (CBX7) within the PRC1, which interacts with ANRIL, has been demonstrated in prostate cancer tissues. In prostate tissues, ANRIL binding with CBX7 has been shown to be crucial for suppression of the INK4b/ARF/INK4a locus (14). Moreover, the rs4977574, rs1333048, and rs10757278 within ANRIL have been shown to be associated with prostate cancer risk in Iranian population (29).
3.3. ANRIL in Hepatocellular Carcinoma (HCC)
The assessment of ANRIL expression in 77 HCC tissues and corresponding normal tissues have shown its up-regulation in HCC tissues and correlation of its transcript levels with tumor size and Barcelona Clinic Liver Cancer (BCLC) stage. Its silencing could suppress cell proliferation and invasion and trigger cell apoptosis both in vitro and in vivo. Moreover, functional studies showed the role of ANRIL in suppression of Kruppel-like factor 2 (KLF2) transcription in HCC cells by recruitment of PRC2 to the KLF2 promoter region (42). More recently, in vitro studies have shown a negative correlation between ANRIL and miR-122-5p expressions in HCC tissues, which was confirmed through the detection of directly interaction between ANRIL and miR-122-5p. More importantly, in vivo studies showed the effect of ANRIL knockdown in suppression of tumor growth (26).
3.4. ANRIL in Lung Cancer
ANRIL is over-expressed in H1299 cancer cells compared to normal fibroblasts. Its silencing increased p15 expression with no effect on p16 or ARF expression, led to cell-cycle arrest at the G2/M phase and suppressed proliferation of these cells (43). Besides, its knock-down in A549, SPC-A1, NCI-H1650 lung cancer cells suppressed cell proliferation, migration, and invasion in vitro (44). In addition, its silencing in NSCLC cells could weaken cell proliferation and trigger cell apoptosis both in vitro and in vivo. Its effect on decreasing p15 expression in PC9 cells was exerted through silencing of KLF2 and P21 transcription (23). The expression of lncRNA ANRIL has been up-regulated by heparin-binding growth factor secreted from cancer-associated fibroblasts (CAF) in lung tumors. ANRIL is also involved in proliferation, apoptosis, and cisplatin resistance of the tumor cells via modulation of the drug transporters MRP1 and ABCC2 (45). The physical interaction between c-Myc and ANRIL has also been validated in vitro. The effect of ANRIL silencing in suppression of NSCLC cell proliferation potentiate this lncRNA as a putative therapeutic target for patients with NSCLC (24). However, a single study in lung cancer H460 cells indicated ANRIL as an lncRNA responsible in anti-tumorigenesis caused by PLD inhibition and suggested the addition of ANRIL into PLD inhibition as a novel therapeutic modality for controlling lung cancer (46). The discrepancy between these two studies might be due to the difference in cultured cells or other biological variables. The results of clinical studies are in line with oncogenic role of this lncRNA. For instance, the expression level of ANRIL has been shown to be elevated in NSCLC tissues and lung cancer cells than in adjacent non-tumor tissues and normal human bronchial epithelial cells. Such over-expression in clinical tissues was associated with higher TNM stage, advanced lymph node metastasis as well as poor overall survival (23, 44).
Moreover, in a population of 467 Chinese patients with lung cancer treated with platinum-based chemotherapy, ANRIL rs1333049 was a predictor of both severe overall toxicity and severe gastrointestinal toxicity (22). Besides, ANRIL rs10120688 and rs1333049 were associated with response to platinum-based chemotherapy in patients with adenocarcinoma and small-cell lung cancer, respectively, in 498 Chinese patients with lung cancer (25).
3.5. ANRIL in Cervical Caner
ANRIL over-expression has been detected in HeLa cancer cells compared to normal fibroblasts. Its silencing increased p15 expression with no effect on p16 or ARF expression, led to cell-cycle arrest at the G2/M phase and suppressed proliferation of these cells (43). Moreover, ANRIL expression has been elevated in cervical cancer tumors compared with non-cancerous tissues (34, 47). The role of this lncRNA, as a sponge for miR-186, has been confirmed by both bioinformatics tools and luciferase assay. Consequently, the ANRIL/miR-186 axis has been suggested as a culprit in the pathogenesis of cervical cancer (34). Moreover, the higher expression of this lncRNA in clinical samples was associated with advanced International Federation of Gynecologists and Obstetricians stage (FIGO) stage, lymph node metastasis, and poor overall survival indicating a role for ANRIL expression as an independent prognostic factor in cervical cancer. ANRIL silencing has suppressed cell proliferation, migration, and invasion of cervical cancer via the inhibition of PI3K/Akt pathway. Consequently, this lncRNA has been suggested as a putative prognostic biomarker and therapeutic target in cervical cancer (47).
3.6. ANRIL in Ovarian Cancer
The expression of lncRNA ANRIL is increased by heparin-binding growth factor secreted from cancer-associated fibroblasts (CAF) in ovarian cancer cells (45). ANRIL expression was up-regulated in epithelial ovarian cancer (EOC) tissues compared with normal controls in association with advanced FIGO stage and high histological grade. Such over-expression has been demonstrated to be an independent prognostic factor for overall survival in EOC. The role of ANRIL in the stimulation of EOC cell proliferation has been confirmed both by in vitro and in vivo. Such role is suggested to be exerted through down-regulation of P15INK4B and up-regulation of Bcl-2 (35).
3.7. ANRIL in Oral Squamous Cell Carcinoma (OSCC)
The expression of ANRIL is increased by heparin-binding growth factor secreted from cancer-associated fibroblasts (CAF) in OSCC. Moreover, its over-expression has been correlated with both high TNM stage and lymph node metastasis in OSCC. On the other hand, its silencing suppressed proliferation, induced apoptosis, and increased cisplatin cytotoxicity of the tumor cells via diminishing the drug transporters MRP1 and ABCC2 (45).
3.8. ANRIL in Esophageal Cancer
The elevated expression of ANRIL has been detected in human esophageal squamous cell carcinoma (ESCC) tissues compared with the corresponding adjacent non-tumor tissues. In vitro studies revealed the role of ANRIL silencing in the enhancement of the expression of p15 (INK4b) and transforming growth factor β1 (TGFβ1) as well as the suppression of cellular proliferation (36). However, the assessment of association between ANRIL rs2151280 in 380 ESCC and 380 cancer-free controls from Chinese Han populations cases revealed no association between this polymorphism and ESCC (48).
3.9. ANRIL in Gastric Cancer
The elevated expression of ANRIL has been demonstrated in gastric cancer tissues of cisplatin-resistant and 5-fluorouracil (5-FU)-resistant patients as well as cisplatin-resistant cells (BGC823/DDP) and 5-FU-resistant cells (BGC823/5-FU). ANRIL silencing in these cells decreased their survival and invasion capability, increased their apoptosis, and down-regulated the expression of multi-drug resistance (MDR) related genes (31).
Ten-eleven translocation 2 (TET2), which converts 5-methylcytosine to 5-hydroxymethylcytosine in DNA, has been shown to bind to the promoter the region of ANRIL and modulate the expression of ANRIL, INK4a, INK4b, and ARF in gastric cancer cells. Besides, ANRIL silencing inhibits the effects of TET2 on gastric cancer cell proliferation and colony formation (49).
Besides, in a cohort of 120 patients with gastric cancer, ANRIL over-expression has been correlated with a higher TNM stage and tumor size being an independent predictor for overall survival. ANRIL silencing decreased cell proliferation both in vitro and in vivo. E2F1 has been demonstrated to be responsible for the induction of ANRIL expression and ANRIL-mediated cell proliferation through the epigenetic suppression of miR-99a/miR-449a in gastric cancer cells (32).
3.10. ANRIL in Colorectal Cancer (CRC)
The expression analysis of ANRIL in 97 paired CRC and adjacent non neoplastic tissue samples have shown its over-expression in CRC samples compared with the adjacent non neoplastic tissues in association with decreased survival rate. Moreover, its silencing in HT29 and RKO human CRC cell lines reduced proliferation while suppressed migration and invasion (33).
3.11. ANRIL in Nasopharyngeal Carcinoma (NPC)
ANRIL over-expression has been demonstrated in advanced-stage cancer in a cohort of 88 NPC patients indicating a role for it an independent predictor of overall survival and disease-free survival. In vitro studies have shown ANRIL effect on the induction of side population cells (SP cells) in NPC. Moreover, ANRIL has a role in the enhancement of glucose uptake for glycolysis, through the regulation of the mTOR signaling pathway (50). The over-expression of ANRIL has been demonstrated in NPC tissues and cell lines. ANRIL silencing in CNE2 and HONE1 cells inhibited proliferation, triggered apoptosis, and improved radiosensitivity of cancer cells. In vitro studies suggested miR-125a as a target of ANRIL in these cells (37). Another study has shown the elevated expression of ANRIL while down-regulation of let-7a in NPC tissues and cells. The effect of ANRIL in down-regulation of miR-let-7a expression has been confirmed by luciferase assay. The role of ANRIL silencing in suppression of tumorigenicity and improvement of cisplatin-induced cytotoxicity has offered a hopeful treatment modality for NPC patients (38).
3.12. ANRIL in Hematological Malignancies
The rs564398 within ANRIL has been shown to be associated with acute lymphoblastic leukemia (ALL) phenotype in an association study enrolling 149 leukemia patients, including Philadelphia positive (Ph (+)) acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) samples. This association might show the ability of some ANRIL polymorphisms to alter ANRIL expression and, subsequently, modify CDKN2A/B expression signatures, which would contribute in atypical cell proliferation and ALL susceptibility (39). Another association study in Iranian patients with acute myeloid leukemia (AML) showed no association between rs1333048, rs4977574, rs1333045, and rs10757278 polymorphisms and AML (51). ANRIL genotyping in 108 multiple myeloma patients treated with high-dose melphalan and subsequent hematopoietic stem cell transplantation (HSCT) have shown the association between the rs2151280 (C/T) genotypes and worse progression-free survival. The TT variant was also associated with elevated ANRIL expression and down-regulation of p15, p14ARF, and p16 compared to the TC/CC variants. Consequently, ANRIL might contribute in melphalan-mediated apoptosis through modulation of p14ARF and subsequent p53 (40).
3.13. ANRIL in Osteosarcoma (OS)
The transcript levels of ANRIL have been elevated in OS tissues compared with the adjacent normal tissues in association with tumor size and patients’ outcome. Si-RNA mediated ANRIL silencing has resulted in the induction of cell apoptosis and suppression of cell proliferation and invasion both in vitro and in vivo. Nude mice injected with siANRIL-transfected MNNG/HOS cells have developed smaller tumors as compared with control group (52), which might highlight the role of ANRIL silencing in cancer control.
4. Conclusions
ANRIL role in epigenetic regulation of expression of its adjacent tumor suppressor genes CDKN2B, CDKN2A, and ARF has suggested its contribution in carcinogenesis. This hypothesis has been supported by the observed over-expression of this lncRNA in a wide range of cancers. However, the associations between ANRIL and CDKN2A/CDKNB/ARF expression seem to be context-dependent. For instance, in the context of prostate cancer CBX7 and ANRIL over-expressions have been correlated with CDKN2A/CDKNB/ARF down-regulation (14). On the contrary, in breast tumors, CBX7 has been shown to be down-regulated, while both ANRIL and CDKN2A/CDKNB/ARF have been over-expressed (16). Such observations have led to the supposition of both tumor suppressive and oncogenic roles for CBX7 based on type of the malignancy, microenvironment, and existence of interacting proteins (16).
In addition, certain SNPs within this gene have been shown to confer cancer risk in distinct populations. However, tissue- or disease-specific functions have been suggested for at least some SNPs (41), which might explain lack of associations with some types of cancer despite significant association with other types of malignancies.
The over-expression of ANRIL in cancer cells promotes cell cycle progression through the alteration of signaling pathways such as Akt pathways or oncogenes such as Myc. In addition, ANRIL has a role in the inhibition of apoptosis and enhancement of cell migration and invasion possibly through the modulation of EMT pathways. Consequently, ANRIL has been shown to influence various aspects of carcinogenesis including the cell cycle, apoptosis, and metastasis. The results of in vitro and in vivo studies support the effectiveness of ANRIL silencing in the inhibition of carcinogenesis-related pathways. However, as any signaling pathway is itself affected by several lncRNAs and miRNAs, ANRIL silencing should be considered only as a single part of a comprehensive treatment modality for the effective eradication of cancer.
Clinical and in vitro studies have shown an intricate interactive network between ANRIL, numerous miRNAs, PRC2/PRC1 subunits, and p15/CDKN2B-p16/CDKN2A-p14/ARF gene cluster (16). Uncovering such interactions would facilitate the design of appropriate targeted therapies for different kinds of human malignancies. The crucial role of ANRIL in conferring MDR in cancer cell as being discovered in gastric cancer (31) suggests the combination of ANRIL targeting therapies with conventional chemotherapeutic regimens as a promising option for cancer treatment.