1. Background
Breast cancer is considered one of the common health problems of women among various populations. Breast cancer that has a high incidence of morbidity and mortality in the world occurs with a medium prevalence among Iranian women (1). In developing countries such as Iran, breast cancer occurs 10 years earlier compared to developed countries (2). This cancer has a complex etiology involving interaction between hereditary, environment, and life style (3).
Folate is a water-soluble necessary vitamin with a key role in metabolism of one-carbon groups that is necessary for biosynthesis, repair, and methylation of DNA (4). Methylenetetrahydrofolate reductase (MTHFR) is a key enzyme catalyzes the re-methylation of homocysteine to methionine by conversion of 5, 10-methylenetetrahydrofolate (5, 10-MTHF) to 5-methyltetrahydrofolate (5-MTHF). The 5-MTHF is the methyl donor for conversion of homocysteine to methionine that is the precursor of S-adenosylmethionine (SAM). The SAM is the universal methyl donor for biological methylation reactions such as DNA methylation. Due to the role of MTHFR in DNA biosynthesis and repair, this gene could be a susceptible candidate gene for breast cancer (5).
The location of MTHFR gene is chromosome 1p36.3 and one of its most common polymorphisms is MTHFR C677T. The C to T point mutation at nucleotide 677 of this gene (Ala222Val, rs1801133) results in a thermolabile enzyme with reduced catalytic activity (6). In the presence of this polymorphism (low activity of T allele) in patients with cancer, the plasma level of homocysteine increases and the DNA methylation reduces (5).
The role of MTHFR C677T variants in susceptibility to breast cancer in different populations has been investigated with controversy (6-11).
According to the recent systematic review, the MTHFR C677T polymorphism is a susceptibility factor for breast cancer among Asians. However, the other polymorphism of MTHFR A1298C might not be a risk factor for breast cancer (6).
2. Objectives
The aim of the present study was to investigate the association of MTHFR C677T polymorphism with breast cancer risk among Kurd population of Western Iran.
3. Methods
3.1. Sample
We studied 100 patients with breast cancer consisted of 99 females and 1 male with the mean age of 49.5 ± 10.2 years (29 - 79 years) and 197 age matched females (51.2 ± 10.9 years, range 22 - 82 years; with α = 0.05 the power of study was 90%). There were 71 individuals (71%) with the age of breast cancer diagnosis ≤ 51 years old. Diagnosis of breast cancer was according to standard clinical, radiological, and histological parameters. Demographic, histopathological, and medical characteristics of patients were obtained from the file of patients in the Kermanshah University of Medical Sciences Hospital. Both patients and controls had Kurdish ethnic background according to the interview with individuals asking about their origin that if belong to the Kermanshah province or other neighboring provinces with Kurdish ethnic background such as Ilam or Kurdistan.
All procedures of the research were accepted and approved by the Ethics Committee of Kermanshah University of Medical Sciences, Iran. All patients and healthy individuals agreed to participate in the study and signed an informed written consent form in accordance with the principles of the Helsinki II Declaration. All individuals were informed about the aim and procedures of the research.
3.2. Genotyping
From each individual, 5 mL blood was taken. Genomic DNA was isolated from leukocytes of EDTA-treated whole blood by using proteinase K treatment followed by phenol-chloroform extraction and ethanol precipitation (12). Using agarose gel electrophoresis (1%), the presence of extracted DNA was confirmed. A Nanodrop spectrophotometer (Thermo Fisher Scientific, Waltham, Massachusetts, USA) was used to determine the concentration and purity of extracted DNA.
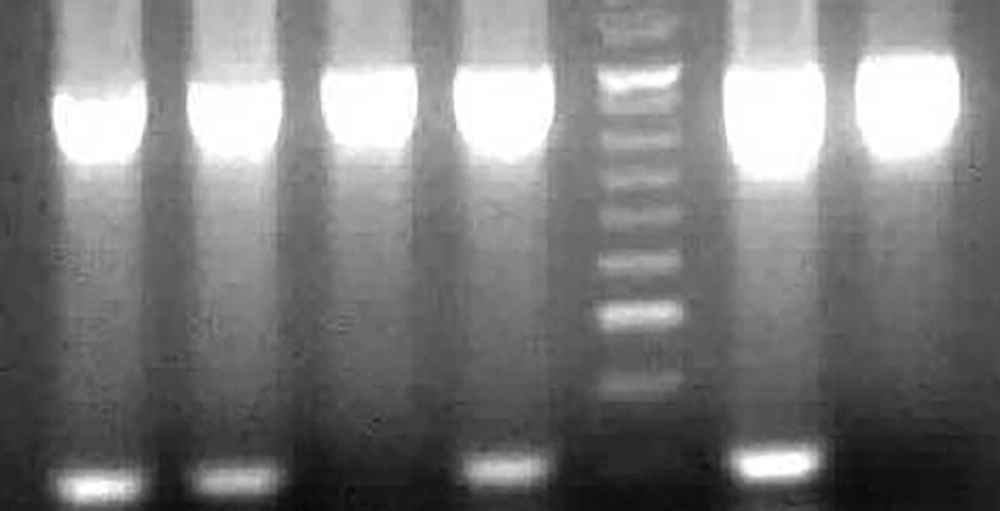
MTHFR C677T was detected, using amplification of a 198-bp fragment in exon 4 of the gene by the 5´-TGA AGG AGA AGG TGT CTG CGG GA-3´forward primer and the 5´-AGG ACG GTG CGG TGA GAGTG-3´reverse primer. Amplification was carried for 40 cycles as follow: 5 minutes in 94°C, 0.5 minutes in 62°C, 0.5 minutes in 72°C, and 5 minutes in 72°C as final extension. Ten to 15 µL of the PCR product was digested with 5 units of the restriction enzyme of HinfI at 37°C overnight. In the presence of C to T conversion, a HinfI recognition sequence is created, which digests the 198-bp fragment into 175-and 23-bp fragments. The HinfI treated PCR products were analyzed by a 3% agarose gel electrophoresis (Figure 1) (13). Genotyping of 10% of samples were randomly repeated to confirm the accuracy of the results. We also tested for possible protein-protein interactions of MTHFR (Figure 2).
The pattern of agarose gel electrophoresis of the MTRR A66G PCR products. Lane 1 depicts PCR product (198 bp), lane 2 indicates heterozygous allele (198, 175, 23 bp), lane 3 shows wild allele (198 bp) and lane 5 shows mutant allele (175, 23 bp). Lane 6 indicates a 50-bp molecular weight ladder.
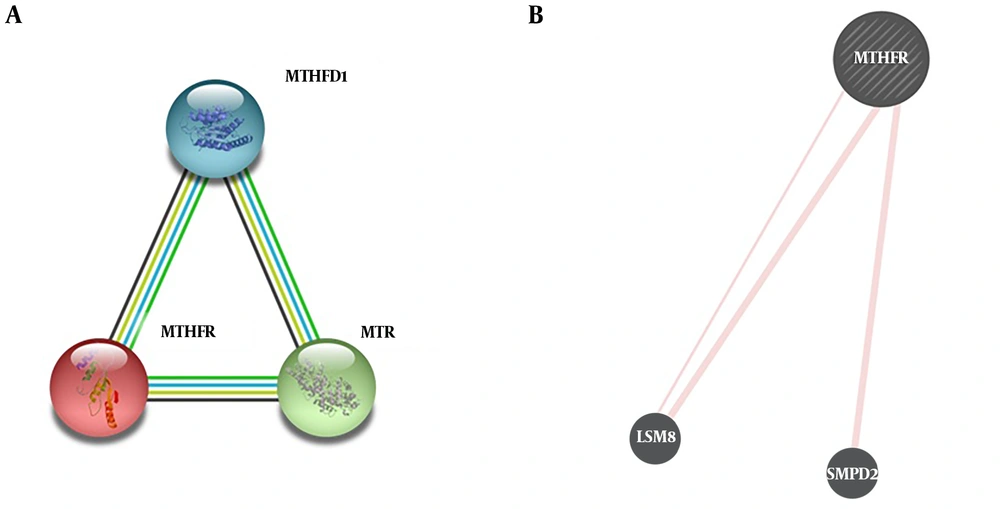
Analysis of protein-protein interactions using STRING (https://string-db.org/cgi/input.pl) and GENEMANIA (https://genemania.org/). A, two top models of proteins interacting with MTHFR according to STRING, and B, predicted physical interactions of MTHFR with LSM8 and SMPD2 according to GENEMANIA.
3.3. Statistics
The frequencies of alleles were calculated by the chromosome counting method. Hardy-Weinberg equilibrium was tested, using a chi-square goodness-of-fit test of SNPstats (14). Using the χ2 method, the significance of the difference in the frequencies of the MTHFR C677T alleles and genotypes between the groups was analyzed. Quantitative characteristics were expressed as mean ± standard deviations. Estimation of relative risk of the disease (odds ratio, OR) and 95% confidence intervals (CI) were measured by logistic regression, using SPSS software. The genotyping analysis was also performed, using multiple inheritance model (co-dominant, dominant, recessive and over-dominant) in SNPstats software (www.snpstats.net).
Quantitative data were compared, using the two-tailed student’s t-test analysis. Statistical significance was assumed at the level of P < 0.05. The SPSS statistical software package version 22 was used for all statistical analyses.
4. Results
Histopathological characteristics of patients with breast cancer are indicated in Table 1.
| Variables | No. (%) |
|---|---|
| Family history of cancer | |
| Absence | 53 (50.5) |
| Presence | 47 (49.5) |
| Stage | |
| I | 13 (19.7) |
| II | 42 (63.6) |
| III | 11 (16.7) |
| Lymph node metastasis | |
| Absence | 21 (28) |
| Presence | 54 (72) |
| Histopathological type | |
| In situ | 1 (1.2) |
| Invasive ductal carcinoma | 73 (89) |
| Invasive lobular carcinoma | 8 (9.8) |
| Estrogen receptor | |
| Positive | 26 (28.8) |
| Negative | 64 (71.2) |
| Progesterone receptor | |
| Positive | 25 (27.7) |
| Negative | 65 (72.3) |
In 49.5% of patients, there was a family history of breast cancer. Among patients, there were 72% with lymph node metastasis. Estrogen and progesterone negative receptors were presented in 71.2 and 72.3% of patients, respectively (Table 1).
No deviation from Hardy-Weinberg equilibrium was found in the patient (P = 0.64) and control groups (P = 0.21) (Table 2).
| N11 (CC) | N12 (CT) | N22 (TT) | N1 (C) | N2 (T) | P Value | |
|---|---|---|---|---|---|---|
| All subjects | 167 | 114 | 16 | 448 | 146 | 0.64 |
| Status = 0-control | 117 | 74 | 6 | 308 | 86 | 0.21 |
| Status = 1-case | 50 | 40 | 10 | 140 | 60 | 0.64 |
The T allele of MTHFR was found in 30% of the patients compared to 27.6% of the healthy controls (P = 0.024) that enhanced the susceptibility to breast cancer by 1.56 times (95%CI: 1.06 - 2.3, P = 0.024) (Table 3). The frequency of MTHFR TT genotype was 10% in patients and 3% in controls (P = 0.008). The presence of TT genotype was associated with a significant increased risk of breast cancer in co-dominant (P = 0.035, OR = 3.90, 95% CI = 1.34 - 11.31) and recessive (P = 0.015, OR = 3.54, 95% CI = 1.25 - 10.03) models of inheritance (Table 4).
| Allele | Cases | Healthy Controls | χ2 | P | OR (95% CI) |
|---|---|---|---|---|---|
| C | 140 (70) | 309 (78.5) | 5.1 | 0.024 | 1.56 (1.06 - 2.3, P = 0.024) |
| T | 60 (30) | 85 (21.5) |
| Model/Genotype | Control | Case | OR (95% CI) | P Value |
|---|---|---|---|---|
| Codominant | 0.035 | |||
| C/C | 117 (59.4) | 50 (50) | 1.00 | |
| C/T | 74 (37.6) | 40 (40) | 1.26 (0.76 - 2.10) | |
| T/T | 6 (3) | 10 (10) | 3.90 (1.34 - 11.31) | |
| Dominant | 0.12 | |||
| C/C | 117 (59.4) | 50 (50) | 1.00 | |
| C/T-T/T | 80 (40.6) | 50 (50) | 1.46 (0.90 - 2.37) | |
| Recessive | 0.015 | |||
| C/C-C/T | 191 (97) | 90 (90) | 1.00 | |
| T/T | 6 (3) | 10 (10) | 3.54 (1.25 - 10.03) | |
| Overdominant | 0.68 | |||
| C/C-T/T | 123 (62.4) | 60 (60) | 1.00 | |
| C/T | 74 (37.6) | 40 (40) | 1.11 (0.68 - 1.81) | |
| Log - additive | - | - | 1.57 (1.05 - 2.33) | 0.027 |
a Values are expressed as No. (%).
Comparing patients with the age of cancer diagnosis ≤ 50 years old and those with the age of cancer diagnosis > 51 years group indicated a higher frequency of MTHFR TT genotype in the latter (20.7%) compared to the first group (5.6%, P = 0.05) that is demonstrated in Table 5.
| MTHFRC677T | Patients ≤ 50 Years Old N = 71 | Patients > 51 Years Old N = 29 | χ2 | P | |
|---|---|---|---|---|---|
| Genotype | |||||
| CC | 36 (50.7) | 14 (48.3) | - | - | |
| CT | 31 (43.7) | 9 (31) | 0.35 | 0.55 | |
| TT | 4 (5.6) | 6 (20.7) | 3.84 | 0.05 |
aValues are expressed as No. (%).
5. Discussion
The presence of MTHFR low activity T allele is associated with increased plasma level of homocysteine and decreased DNA methylation (5).
The present study among a homogenous population with Kurdish ethnic background from Western Iran observed that the frequency of MTHFR TT genotype was significantly higher in patients with breast cancer than healthy individuals that increased the susceptibility to breast cancer. Also, the higher frequency of MTHFR T allele among patients was associated with 1.56 times susceptibility to breast cancer. Further, we detected a higher frequency of mutant genotype of TT among patients > 51 years old than those ≤ 50 years old.
The role of MTHFR C677T polymorphism in susceptibility to breast cancer among different populations is controversial. Among a population from South-Eastern Europe, the MTHFR C677T was related to the risk of breast cancer. The menopausal status of women did not influence the association between the MTHFR gene polymorphisms and the risk of breast cancer (15). In Mexican women, the MTHFR 677TT genotype was a risk factor for breast cancer (16). But, in a large sample of Canadian women, the MTHFR C677T was not found to be a risk factor for breast cancer (17).
Some studies from Asian populations reported the influence of MTHFR C677T variants insusceptibility to breast cancer but with inconsistency. In China, some studies found an association between MTHFR 677TT genotype with enhanced breast cancer risk (6, 7). Among Japanese, an association was reported between MTHFR 677TT genotype with the risk of breast cancer in postmenopausal women (8). Also, in a study from Iran, an association was detected between MTHFR C677T polymorphism with breast cancer risk (10). Further, in a population from India, the MTHFR C677T elevated the risk of breast cancer (5). However, among South-Eastern Asian populations (11) and Syrian women (9), the lack of association between MTHFR C677T polymorphism and the breast cancer susceptibility was reported.
In a meta-analysis conducted by Zhong et al. (18), strong association between MTHFR C677T polymorphism and the risk of breast cancer was detected in a population from East Asia and suggested the risk of breast cancer was significantly associated with women postmenopausal status. However, the meta-analysis did not detect such association in Caucasian population (18). Also, two recent meta-analyses suggested that the MTHFR 677TT genotype is significantly related to the breast cancer risk in Asian populations (19, 20). A more recent meta-analysis indicated that the MTHFR C677T polymorphism is a risk factor for breast cancer in all genetic models and suggested this polymorphism could be a therapeutic target for breast cancer (21). Furthermore, a recent meta-analysis performed by Naushad et al. (22) revealed that the MTHFR C677T is a risk factor among postmenopausal women with breast cancer. The higher risk of breast cancer in postmenopausal women than the premenopausal women could be results from difference in the expression of estrogen receptor in the first group and there might be estrogen receptor gene misregulation in the presence of MTHFR 677TT genotype (8). Among Brazilian women, although the MTHFR C677T polymorphism was not associated with the risk of breast cancer, but it was related to clinical severity of the disease (23). The inconsistent findings of association between the MTHFR C677T and breast cancer risk in different populations may underlie ethnic differences, different lifestyle, and disease prevalence as well as possible limitations due to the relatively small sample size. Wide variation in the MTHFR T allele frequencies among controls in Asians (0.396), among Middle Eastern countries (0.201), in Indians (0.132), among Caucasians (0.326), and among Africans (0.196) might be accounted for the discrepancy in association between the MTHFR C677T polymorphism and the cancer risk in different ethnicities (19).
The protein-protein interactions of MTHFR revealed in addition to currently known interactions with proteins like 5-methyltetrahydrofolate-homocysteine methyl transferase (MTR) and methylenetetrahydrofolate dehydrogenase (MTHFD1), other possible physical interactions have been proposed for MTHFR, including sphingomyelinphosphodiesterase 2 (SMPD2) and U6 small nuclear RNA associated (LSM8). So, it worth further deciphering any functional interaction between MTHFR and the above-mentioned genes within the cell and in pathogenesis of diseases like breast cancer as well.
In conclusion, our study indicated that the MTHFR C677T polymorphism is associated with the risk of breast cancer among a population from Western Iran. Also, our study suggests that MTHFR TT genotype might be a risk factor for breast cancer in postmenopausal women.