1. Background
Since 1980, the deployment of external beam radiotherapy quickly expanded as a therapeutic modality in patients with advanced breast cancer. Due to the advancement of various treatment methods, radiotherapy following breast conserving surgery (BCS) is standard method in order to control and prevent the recurrence occurrence in patients with advanced breast cancer, which became equivalent to radical mastectomy (1-3).
This change in treatment method is arising from medical perspective in order to improve the quality of life and decreasing surgical morbidity and complications rate while increasing the quality of treatment (4, 5). In patients with proper breast preservation, radiotherapy accepted as a complementary therapeutic component after BCS (6, 7).
Many studies show good results of deploying the method of accelerated partial breast irradiation in comparison with standard external beam radiotherapy (EBRT) to minimize surgical complications and improve the quality of treatment process (8-13).
According to the retrospective studies, which were performed on more than 30 000 patients, most of the patients and physicians tend to use intraoperative radiotherapy technique (14).
In the past, with this notion that invasive lobular carcinoma (ILC) is a lesion with multifocal and diffuse pattern of spreading, BCS was not recommended (15). Meanwhile, in later studies with the same surgical technique, the local recurrence rate was almost equal with when the radiotherapy was performed after the surgery (16-18).
In another study performed on 45 German-Australian patients, who had ILC and undergoing BCS and accelerated partial breast irradiation (APBI), there was no significant difference in 4-year recurrence. Based on this evidence and studies that demonstrated the equivalence of this method in two types of ILC and IDC pathologies, later designs, BCS, and APBI were accepted as a valid method for later studies (19-21).
Still, there are many challenges about the appropriate ness of using APBI for these patients because of having favorable outcomes in ILC patients (22, 23). This is due to the existence of older patients with higher levels of hormones and less proliferation in group of ILC compared with IDC patients (22, 23).
According to the literature, the rate of breast cancer local recurrence after electron IOERT was 0.02% per person- month, with an adjusted 5-year recurrence rate of 2.7% (24, 25). These findings confirm the recent guidelines published by American Society of Radiation Oncology (ASTRO), supporting the use of electron IOERT for low-risk patients (24, 25).
In another study, 2 800 patients were evaluated and 21% of them underwent the BCS and IOERT. Intraoperative radiotherapy resolved the problem of patients, whose homes were far from the radiotherapy centers or located in remote villages, border cities, or mountainous areas and there was impossibility of daily radiotherapy being performed for them (26).
In a recent study, it has been shown that APBI is an acceptable modality in ILC (26).
2. Methods
Between August 2013 and September 2017, 968 patients, who were referred to Cancer Research Center of Shahid Beheshti University of Medical Sciences with invasive breast cancer, were treated with breast-conserving surgery and radiotherapy. Of these, 426 patients received a tumor bed boost with IOERT during lumpectomy (58 patients with pure invasive lobular carcinoma, 239 patients with pure invasive ductal carcinoma, and 129 patients with other diagnoses). The conventional EBRT was delivered to 542 patients (24 patients with pure invasive lobular carcinoma, 418 patients with pure invasive ductal carcinoma, and 100 patients with other diagnoses). The patients were followed up to 49 months. A comprehensive list of clinical and pathologic features was evaluated for all the 3 group patients (pure ILC and IDC and conventional EBRT).
According to the result of biopsy sample, which was obtained from all patients, they were candidate to undergo BCS. All pathologic and biologic characteristics of patients were defined before the surgery. A multidisciplinary team, including a cancer surgeon, a radiotherapist, and a physicist was present in the operating room. After the tumor removal, clearness of margins cells was defined, using frozen section technique; according to our criteria, electrons (with dose of 12 Gray (Gy)) in boost were radiated to the healthy margins, using LIAC linear accelerators, (Sordina IOERT Technologies S.p.A., Italy), while the patient was anesthetized.
In clinically negative axilla, the axillary lymph nodes were checked, using sentinel biopsy and in the case of axillary lymph node involvement, dissection was performed; this is very important in determining the type of radiotherapy.
Acording to IRIORT (Iranian intraoperative radiotherapy) working group criteria shown in Table 1, patients who were candidates for radical IOERT were excluded from this study
| Factors | Suitable | Possible | Contraindicated |
|---|---|---|---|
| Age, y | ≥ 45 | 40 - 44 | < 40 |
| Tumor size, cm | < 3 | 3 - 3.5 | > 3.5 |
| Margin | Negative | Negative | Positive |
| Grade | 1 and 2 | Any | - |
| LVI | Negative | Any | - |
| ER,PR | Positive | Any | - |
| Multicentricity | No | No | Yes |
| Multifocality | No | Yes | - |
| IDC | Yes | Yes | - |
| ILC | Yes | Yes | - |
| Pure DCIS, cm | ≤ 3 | 3 - 4 | > 4 |
| EIC, | < 25 | ≥ 25 | Diffuse |
| HER2 | Any | - | - |
| LCIS associated | Any | Any | Any |
| Nodal status | Negative | Negative | Positive |
| Axillary surgery | SLNB | SLNB or ALND | - |
| Neoadjuant Th. | Not allowed | Not allowed | If used |
Systemic therapy, chemotherapy, and/or endocrine therapy were appropriate for the selected patients. All patients were followed-up on a regular basis to assess local disease control and survival. Clinical examinations were performed at least every 6 months and mammograms and ultrasound were required yearly if indicated. Magnetic resonance imaging (MRI) was not routinely performed. This review was approved by the Ethics Committee of Cancer Research Center of Shahid Beheshti University of Medical Sciences.
2.1. Statistical Methods
In this study, we evaluated the incidence of local and distance (metastasis) recurrence in all patients, whichever occurred first and was evaluated by the Kapla-Meier method. The log-rank test was used to assess the difference in the incidence of IBTRs and distance recurrence in patients with pure IDC and pure ILC. We obtained 4-year event rates with respective 95% confidence intervals (CIs) from actuarial survival analysis.
In the multivariate analysis, estrogen receptor (ER), progesterone receptor (PR), and Human epidermal growth factor receptor 2 (HER2) expression were represented by molecular subtype. A “reduced” multivariate model was, then, built, using only those parameters that were statistically significant in a fully adjusted model. The multivariate-adjusted hazard ratio (HR) for the risk of IBTR and distance (metastatic) recurrence in patients with ILC (IOERT and EBRT) and IDC was compared in different patients’ subgroups. In addition, a propensity score matching analysis was performed in the group of ILC, in which patients were matched with an equivalent group of IDC. The propensity score was built via a multivariate logistic regression model, considering all the variables listed in Table 2; all analyses were performed with SPSS software (version 22).
| Characteristic | ILC, N = 82 | IDC, N = 239 | |
|---|---|---|---|
| Patient Series, No. | IOERT, N = 58 | EBRT, N = 24 | IOERT, N = 239 |
| Age, y | |||
| ≤ 45 | 18 (31) | 10 (41.6) | 99 (41.4) |
| 46 - 55 | 19 (32.7) | 10 (41.6) | 78 (32.6) |
| 56 - 65 | 14 (24.1) | 4 (16.6) | 32 (13.3) |
| ≥ 65 | 3 (5.1) | 0 (0.0) | 19 (7.9) |
| Missing | 4 (6.8) | 0 (0.0) | 11 (4.6) |
| Pathologic size, cm | |||
| < 2 | 10 (17.2) | 4 (16.6) | 86 (35.9) |
| 2 - 5 | 33 (56.8) | 14 (58.3) | 96 (40.1) |
| > 5 | 10 (17.2) | 5 (20.8) | 36 (15) |
| Missing | 5 (8.6) | 1 (4.1) | 21 (8.7) |
| Nodal (N) status | |||
| N0 | 20 (34.4) | 10 (41.6) | 92 (38.4) |
| N1 | 21 (36.2) | 5 (20.8) | 103 (43) |
| N2 | 12 (20.6) | 5 (20.8) | 26 (10.8) |
| N3 | 4 (6.8) | 2 (8.3) | 15 (6.2) |
| Missing | 1 (1.7) | 2 (8.3) | 3 (1.2) |
| Tumor grade | |||
| G1 | 10 (17.2) | 7 (29.1) | 14 (5.8) |
| G2 | 35 (60.3) | 7 (29.1) | 110 (46) |
| G3 | 12 (20.6) | 6 (25) | 110 (46) |
| Missing | 1 (1.7) | 4 (16.6) | 5 (2) |
| Lymphovascular invasion | |||
| Absent | 38 (65.5) | 18 (75) | 85 (35.5) |
| Present | 20 (34.4) | 5 (20.8) | 154 (64.5) |
| Missing | 0 (0.0) | 1 (4.1) | 0 (0.0) |
| Estrogen receptor | |||
| Absent | 6 (10.3) | 12 (50) | 69 (28.8) |
| Present | 51 (87.9) | 10 (41.6) | 161 (67.3) |
| Missing | 1 (1.7) | 2 (8.3) | 9 (3.7) |
| Progesterone receptor | |||
| Absent | 5 (23.0) | 10 (41.6) | 69 (28.8) |
| Present | 53 (76.9) | 11 (45.8) | 160 (66.9) |
| Missing | 0 (0.0) | 3 (12.5) | 10 (4.1) |
| HER2 | |||
| Not overexpressed | 50 (86.2) | 16 (66.6) | 163 (68.2) |
| Overexpressed (+++) | 4 (6.8) | 6 (25) | 43 (17.9) |
| Missing | 4 (6.8) | 2 (8.3) | 33 (13.8) |
3. Results
The follow-up period was 49 months (August 2013 to September 2017). Table 2 summarizes the clinical and pathologic characteristics by histology of breast cancer (pure ILC and IDC) treated with boost intraoperative electron radiotherapy (IOERT), and (pure ILC) conventional boost external beam radiotherapy (EBRT).
The median age of patients IOERT was 50 and EBRT 46.3 years for the ILC group and 47 years for the IDC group. Compared with the IDC group, patients with ILC were older (56 - 65 years, 24.1% ILC IOERT, and 16.6 ILC EBRT vs. 13.3% IDC IOERT) with more lymph node involvement (pN2, 20.6% ILC IOERT, and 20.8 ILC EBRT vs. 10.8% IDC IOERT), less grade (grade 3, 20.6% ILC IOERT, and 25% ILC EBRT vs. 46% IDC IOERT), and less peri tumoral vascular invasion (34.4% ILC IOERT and 20.8 ILC EBRT vs. 64.4% IDC IOERT).
Patients with low risk of recurrence, who had desirable criteria to receive radical dose of IOERT (suitable for radical dose), were excluded from this study and patients of both groups of ILC and IDC had been radiated based on the same technical parameters. All patients, who had received radiation, had been diagnosed with pure ILC and IDC and none of them had mixed ILC/IDC tumor. It is worth noting that in a parallel study, there was no significant difference between ILC and mixed ductal and lobular patients, who underwent BCS and external radiotherapy (27).
Among 58 patients in the ILC group treated with IOERT boost and 24 patients in the ILC group treated with EBRT boost, none of the patients experienced a relapse either locally or distantly, with a 4-year survival rate of 100%. But in the IDC group treated with IOERT boost, the survival rate was 97% (232 out of 239 patients). In the IDC group, 2 patients experienced IBTR and 5 patients progressed to metastatic disease (2 patients had lung involvement and 3 patients had brain and bone involvement).
The average interval between the time of disease diagnosis to the time of local and distant recurrence occurrence was 15 and 20 months, respectively. During the follow up period, no patient of ILC group (IOERT and EBRT) suffered from contralateral breast carcinoma and none of them experienced local and distant recurrence simultaneously. The odds ratio between two rows is defined as:
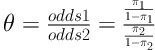
According to Agresti, the sample odds ratio θ equals 0 or ∞ if any of entries has zero frequency, and it is undefined if both entries in a row or column are zero. He preferred the below estimator for the case that there is a cell or cells with zero frequency,
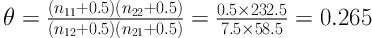
When 0 < θ < 1, the odds of recurrence are lower in row 1 (ILC Group) than in row 2 (IDC group). So, the odds of recurrence in IDC group are 3.77 (
4. Discussion
Breast conserving surgery and its following radiotherapy is an accepted therapeutic method for patients with breast cancer. In the past decades, ILC patients underwent mastectomy, but today, BCS and its following radiotherapy is a confirmed treatment technique for these patients (28). Of course, due to the nature of multifocal lesions in ILC, The instructions raised this issue more cautiously (27, 29). However, in recent studies, higher recurrence in ILC patients has not been seen (26, 30).
In the present study, we evaluated the local and distant recurrence in patients with breast cancer and with pathology of pure ILC and pure IDC. All patients with the high-risk of recurrence in both groups underwent BCS and radiotherapy. In the group of pure ILC (IOERT boost or EBRT boost), all patients have been cured and there were no symptoms of local or distant recurrence during 4-year follow-up. In the group of pure IDC, 2 and 5 patients experienced local and distant (metastasis) recurrence (Table 3).
| Variable | ILC, N = 82 | IDC, N = 239 | |
|---|---|---|---|
| IOERT, N = 58 | EBRT, N = 24 | ||
| Disease-free | 58 (100) | 24 (100) | 232 (97) |
| IBTR | 0 (0) | 0 (0) | 2 (0.8) |
| IBTR and distance | 0 (0) | 0 (0) | 0 (0) |
| Distant metastasis | 0 (0) | 0 (0) | 5 (2) |
| Contralateral breast cancer | 0 (0) | 0 (0) | 0 (0) |
a Values are expressed as No. (%).
There is no similar study on evaluating local and distant recurrence in patients with breast cancer and high-risk of recurrence. However, in patients, who underwent radical IOERT (low-risk of recurrence) in early stage, Leonardi et al. compared two types of pathology, who have received full dose IOERT with electrons and found that local recurrence in ILC group was higher than it in IDC group (27).
Some literatures have limitations such as follow-up periods (31). In the current study, despite the clinical, epidemiological, and pathological factors such as tumor size and immuno histo chemistry (IHC) tests, due to lack of recurrence in ILC of the discussed groups, there was no possibility to compare the data accurately and statistically.
Lack of local/distant recurrence in patients with breast cancer and with pathology of ILC (IOERT boost and EBRT) may lead to use IOERT modality as a boost; it has no inferiority in comparison with those who suffered from breast cancer with IDC pathology. According to an unpublished study in cancer research center of Shahid Beheshti University of Medical Sciences on biological effects and processes of electron IOERT as a boost dose, which was induced immediately and after 24-hours from a 12Gy radiation, the effect lasted over the time. This study showed not only the effect of 12Gy boost had no inferiority compared with a higher dose as 21Gy radical, but also it had more biological effects in the induction of cellular and molecular positive effects. It could show the local and systemic effects of radiation on tissue and may be affected due to help improving immune system to control distant recurrence; our epidemiological data confirm this status data as well.
4.1. Conclusions
We suggest that boost IOERT can be considered as a radiotherapy modality in ILC similar to IDC. The strength of our study was the non-selectivity of patients based on the risk factors of recurrence. In other words, we evaluated both local and distant recurrence in patients, while the follow-up period was a limitation in our study. This result suggests that IOERT technique deployment had no inferiority compared with the control group.
Although this conclusion cannot strongly validate this hypothesis at present, but we cannot ignore the effects of high-dose radiation on healthy tissue surrounding the tumor. Therefore, we recommend more studies should be done with a larger sample size and longer follow-up period.