1. Background
Antineoplastic drugs as chemotherapy agents are used for various therapeutic purposes (1-4). Despite the benefits of such drugs for patients (5), their use in hospitals has negative implications for the health of hospital employees, especially oncology personnel (6). According to American Society of Hospital Pharmacists (ASHP) and National Institute for Occupational Safety and Health (NIOSH) guidelines, hazardous drugs, such as antineoplastic drugs, should be administered under certain drug safety provisions when they are received, stored, prepared, administered, or disposed (6, 7). Recent efforts to decrease or eliminate workplace contamination include the use of engineering controls such as robotic systems (8, 9), closed system drug transfer devices (CSTDs) (10-13), and compounding aseptic containment isolators (CACIs) (14). According to the instruction for work-related hazardous agent measurement, employers are responsible for the safety of their employees at risk; in fact, periodical monitoring of employees for their possible occupational exposures have been considered (15). Traditionally, occupational exposures are monitored periodically through personal environmental exposure. Biological monitoring has been regarded as more comprehensive monitoring due to consideration of all exposure routes through respiratory, skin, and gastrointestinal absorption (16). In recent years, biological monitoring was reported as the best method for monitoring hazardous exposure to cytotoxic drugs (17). Considering the findings relating to the measurable quantity of hazardous drugs in the biological specimen of oncology personnel observing the safety protocol, concern for such drug-handlers has been raised only recently. In addition, even after the implementation of safety considerations, significant concentrations of some hazardous drugs have been reported in the urine of health service staff, who prepare or administer these drugs (18-22). All clinical and non-clinical staff have possible exposure to drugs in case of vapour, dust, or skin contact with contaminated surfaces of pharmaceutical spills collected during the preparation, administration, or disposal of pharmaceutical wastes (23). Occupational exposure of drug-handlers was reported through respiring airborne aerosols or skin contact with the drug during administering to patients or touching contaminated surfaces and disposal of wastes (24-26). Exposure to antineoplastic drugs is possible through inhalation, skin contact, skin absorption, and digestive or injection (27). CPA is one of the most dangerous antineoplastic drugs, which is widely used for the treatment of leukaemia and lymphoma, many types of bladder, ovarian, breast, lung, endometrium, neuroblastoma, retinoblastoma cancers, Ewing’s sarcoma, and Wilm’s tumour (28). The results of a study conducted by Villarini et al. showed that among 40 people under biological monitoring, detectable levels of CPA were measured in urine samples after working shift in 7 nurses (17.7 % of all samples). CPA in urinary concentrations was within the range of 0.1 to 0.2 micrograms per litre, while one of the samples had concentrations of 1.2 micrograms per litre (29). Sessink and Bos have pointed out in their study, despite the observance of safety protocols by health workers in 12 studies, detectable CPA levels were measured in the urine of 11 groups of the studied healthcare workers (25). In another study carried out by Harrison 13 out of 20 healthcare workers demonstrated different quantities of 6 different drugs (cyclophosphamide, methotrexate, ifosfamide, apiropsin and cisplatin/carboplatin) in their urine (30). There was not any studies about the biological monitoring of Iranian oncology personnel’s.
2. Objectives
Considering the importance of safeguarding the health oncology personnel, the aim of this study was to examine the validation parameters of the method developed by Sessink et al. (31) for biological monitoring the oncology personnel and also to biomonitor the exposure of the Iranian health workers through the measurement of urinary CPA as the biomarker of the exposure at the two major hospitals in Tehran.
3. Methods
3.1. Study Design
This cross sectional experimental study was conducted in two hospitals in Tehran, Iran from September 2015 to January 2016. The two hospitals included 3 preparation rooms, 49 inpatient beds, and 10 outpatients working at two oncology wards.
The participants of the study were pharmacy technicians, nurses, and auxiliary workers with at least 1 year of employment (Table 1). They consented to participate in the study by signing form prior to seeking their demographic information and their work conditions in a questionnaire. The sampling method was convenience sampling. The exclusion criteria included those with chemotherapy history that taking CPA in the past 12 months. According to the previous studies and considering α = 0.05, β = 0.9 and “
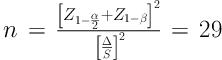
| Hospitals | Preparation Rooms | Inpatient Beds | Outpatient Stalls | Preparation Technicians | Nurses | Auxiliary Staff | Total |
|---|---|---|---|---|---|---|---|
| A | 2 | 32 | 10 | 4 | 12 | 10 | 26 |
| B | 1 | 17 | 0 | 2 | 10 | 7 | 19 |
| Total | 3 | 49 | 10 | 6 | 22 | 17 | 45 |
Considering the loss of 10% of the participants during the course of the study, 32 staff in the oncology ward will be selected as the study group. Urine specimen were obtained at the end of the work shift and stored at -20ºC until analysis.
3.2. Analytical Method and Measurements
All chemicals, solvents and standards were of analytical grade and used as supplied.
3.3. Preparation of Standard Solutions of CPA
A stock solution of CPA was prepared at a nominal concentration of 0.1 mg/mL by weighting a pure powder (Baxter, Germany) and dissolving the weighted amount in methanol. A stock solution of IFO to be used as internal standard was prepared at a nominal concentration of 0.1 mg/mL by weighting a pure powder (Baxter, Germany) and dissolving the weighted amount in methanol.
Working solution of CPA in the concentration range of 0.02 - 50 µg/L was prepared by diluting a stock solution of CPA in urine that was obtained from a healthy man, who had no contact with CPA. IFO was added as an internal standard at a concentration of 20 µg/L. The prepared standard solution and samples from the workers were stored at 4ºC until the analysis.
3.4. Measurement of CPA in Urine
This method is an adaptation of that described by Sessink et al. (31) for the analysis of CPA in urine specimen (32). Briefly, 5-mL urine samples, both CPA standards and urine specimen were spiked with the IFO as an internal standard at concentration of, 20 µg/L. All standards and urine specimen extracted twice with 10 mL each of analytical grade diethyl ether (Merck Co.). The combined organic layers were dried under pure nitrogen gas (99.9 %, Mahan Gas Co.), re-dissolved in 100 µL of analytical grade ethyl acetate (Merck Co.) and dried under shower nitrogen gas again and were dissolved in 100 µL of analytical grade toluene (Merck Co.) for analysis.
Measurement was accomplished by capillary gas chromatography with electron capture detection (GC-ECD) in a GC-ECD model No. 17A (Shimadzu, Japan). Separation was accomplished in a BP5 (SGE Analytical Science Co.) capillary column. The carrier gas was nitrogen 99.9995% (Mahan Gas Co.) and the column flow was 1.8 mL/min. The GC temperature gradient was as follows. At injection, the oven was held at 100ºC for 2 min, followed by a first gradient of 6ºC/min to 160ºC. After 1 min, the temperature was increased with a gradient of 8ºC per min to the final temperature of 250ºC. Total run time was 25 min. Urine specimen and standards were injected at 1 µL, in the split-less mode (vent time 60 sec). Elution of CPA occurred at 17.5 min, of IFO (internal standard) at 16.7 min.
3.5. Validation of CPA Measurement
Since the method of this study was an adapted form of a method by Sessink et al. (31). The US Food and Drug Administration (US-FDA) guideline was used for validation of the method presented. The validation parameters include the lower limit of detection (LLOD as 3 times the signal of the baseline noise at the elution time of CPA), the linear concentration range, accuracy, precision, and lower limit of quantification (LLOQ, as the lowest concentration level that yields a peak area 10 times that of the LLOD peak), intra- and inter-day variability, stability of spiked urine samples. Quality control (QC) stocks were prepared as spiked urine samples at low (0.1 µg/L), medium (30 µg/L), and high (50 µg/L). Three replicates of the samples at each concentration were evaluated on the same day for intra-day precision, while repeated analyses at each concentration of the samples for 3 times a day over 5 successive days were carried out for inter-day precision.
Recovery was assessed in the 3 CPA QC-samples. Stability was assessed in the low- and high-level QC-samples for a period of 1 to 10 days, while they are kept in a refrigerator at -4ºC.
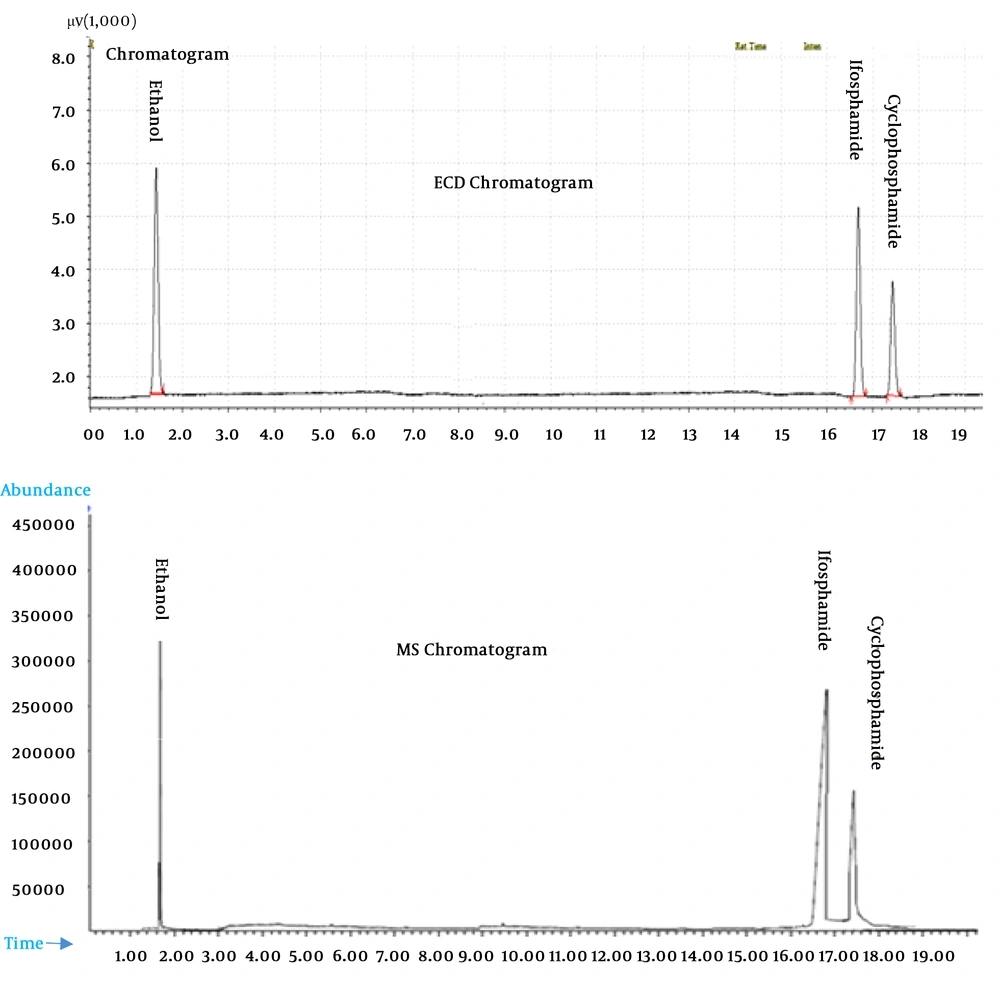
The identity of CPA and IFO peaks in the GC-ECD traces was confirmed by gas chromatography-mass spectrometry GC-MS (Agilent 5975c), using the same capillary column and chromatographic conditions, according to the procedure suggested by Feyerherm et al. (33).
3.6. Statistical Analysis
The Microsoft Excel 2007 was used to calculate the method parameters. The correlation between urine and previous author’s (34) skin monitoring results was examined with Pearson’s rank correlation coefficient. The P = 0.050 is considered significant. Data analysis was performed with SPSS computer software V. 21.
3.7. Ethical Considerations
The study was approved by the research Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (code: 13580). Informed consents were obtained from all participants and each one received a code to be unknown.
4. Results
4.1. Analytical Method
The development of the analytical method afforded a performance as described in Table 2. In particular, the capillary column afforded a good separation of CPA and IFO from the background material of the organic extract, as witnessed by the confirmation of the GC peaks in the mass spectrometer (Figure 1). The validation parameters of the analysis of CPA in the urine specimen are reported in the Table 2. Generally, the performance the adapted method using BP5 capillary column capillary column demonstrated adequate chromatography for CPA and IFO as internal standard from the other organic compounds in the urine specimen, as witnessed by the confirmation of the GC peaks in the mass spectrometer (Figure 1).
| Parameter | Validation Data |
|---|---|
| Calibration range, µg/L | 0.5 - 50 |
| R2 | 0.995 |
| Linear range, µg/L | 0.5 - 50 |
| Lower limit of detection (LLOD), µg/L | 0.2 |
| Lower limit of quantification (LLOQ), µg/L | 0.5 |
| Recovery, % | 84.1 |
| Precision (range of coefficient of variation), % | |
| Intra-day | 8 - 10.5 |
| Inter-day | 5 - 14 |
| Stability | 8 days at -4ºC |
4.2. Biological Monitoring
The mean and the range of the oncology personnel’s age and work history were 29.75 (22 - 40) and 3.12 (1 - 7) years, respectively.
The results of CPA measurement in the urine of the oncology personnel at two hospitals were presented in Table 3. As shown, 10 out of 32 urine samples taken from oncology personnel at hospitals A and B demonstrated higher-than-LLOD CPA concentration. From the 6 positive samples at hospital A, 5 samples belonged to oncology nurses and 1 sample was from cleaning crew. However, all positive samples were in hospital B belonged to oncology nurses. The highest CPA concentration (21.4 µg/L) was from a nurse working in hospital B.
| Hospital | No. of Sample (Number of Positive Samples > 0.2 µg/L) | Mean ± SD | Range |
|---|---|---|---|
| A | 22 (6) | 9.53 ± 7.33 | 0.62 - 19.18 |
| B | 10 (4) | 11.98 ± 9.75 | 0.52 - 21.4 |
5. Discussion
Validation processes were used for the method of GC-ECD to ddetermination of CPA in urine of exposed the oncology personnel of two hospitals. This method was linear for CPA in the range of 0.5 to 50 µg/L. A comparable LLOD were found by Sessink et al. (31) for the CPA analysis. However, in this study we used the ECD detector.
In an author’s previous study (34), skin sampling was taken from all personnel (N = 32) that participated in this study. The highest concentration of CPA in the dermal sample (144.35 ng/wipe) was detected on the hands of an employee, who worked in preparation room No. 1 at hospital A. CPA was detected in the skin samples of oncology personnel at two hospitals within the range of 83.1 to 144.35 ng/wipe. The result of the statistical analysis of the correlation between skin samples obtained from the author’s previous study (34) and the urine samples taken from the same personnel in this study were significant (P = 0.002, R = 0.67). Based on an observational field study in our hospitals, the majority of the personnel do not follow the guidelines and procedures recommended by international institutions and do not use the recommended safety equipment. This study was to pursue the visionary idea of scholars regarding the promotion of biological monitoring in the risk evaluation of occupational exposure to hazardous chemicals for better management in future (9, 17). Occupational exposure to antineoplastic drugs, such as CPA, could be detrimental to the health of oncology personnel (6), and their biological monitoring was recommended recently by Jakubowski (17). Occupational exposure to CPA drug was reported to be taking place through respiratory and skin routes (35-37). The authors of this study have recently published articles measuring the external exposure of the same oncology personnel’s exposure to CPA through the skin route (34, 38). In accordance with our previous data for the external occupational exposure to CPA through skin contamination, and also with the data from the urinary concentration of the same group of personnel in the present study, significant statistical correlation was observed (P < 0.05). These phenomenon could signify the skin absorption of CPA by personnel, who handle CPA drug in the oncology department. Similar to our findings about the system absorption of CPA, Sessink et al. (19) also reported this phenomenon in another study, which reported the urinary concentrations of CPA of the oncology personnel without appreciable exposure to CPA in their ambient air, but with considerable surface contamination with the drug at various workstations. Based on a guideline given by NIOSH (6) on chemical safety of antineoplastic drugs, the majority of oncology personnel in this study did not follow the recommended safety equipment and procedures for administering drugs to patients with cancer. Contrary to the results of this study about the urinary concentration of CPA in the oncology personnel with having potential external exposure of through breathing air and skin, another similar study was reported with the preparation of the CPA drug with the aid of a robotic system and without appreciable occupational exposure to the technicians (9). The oncology personnel in this study were examined for their compliance with the safety protocols according to the criteria given by the NIOSH (6) and the ASHP (7). According to our findings, our oncology personnel were not trained for their work-tasks and did not have the proper personal safety equipment and engineering control measures. However, in another similar study with full implementation of control measures, insignificant urinary CPA concentrations were reported for the oncology personnel (9). Generally, the positive role of work practice due to proper training of oncology personnel was clearly demonstrated by Turk et al. (39), which indicated the influence of the knowledge on their attitude of implementing the safety measures while handling cytotoxic drugs. Other authors have also stated that the lack of knowledge could influence their behaviour (40, 41).
5.1. Conclusions
This study succeed its purpose to demonstrate the ability of a comparatively simple and convenient analytical method based on liquid-liquid extraction and gas chromatography with the ECD to measure CPA in the urine of hospital workers, who manipulate anti-cancer drugs. Considering that we observed a higher frequency and higher exposure levels of the oncology personnel, compared to their colleagues abroad, we believe that the examined hospitals deserve to better organize interventions to protect the health of oncology technicians and nurses, such as periodic training, better control measures, and periodic re-checking of the efficacy of prevention measures.