1. Context
Cancer is a chief public health issue all around the world, which is considered as a lethal disease in most regions like the third leading cause of death in Iran and the second in United States (1, 2). Studying the cellular basis of cancer, most of the time an error in replication leads to a cancerous cell (3). On the molecular basis, cancer is a result of chromosome instability, DNA-repair defects, or aberrant DNA methylation (4, 5). Current evidence supports the idea that chemical exposures are associated with DNA-methylation and DNA-repair mechanisms, and, as a result, they could get the expression processes under influence and affect human health (6-9). Therefore, by studying the pathology of cancer, a considerable association between the exposure to particular chemicals and the onset of malignancy can be witnessed (5).
Disinfectants are the common chemicals used to remove pathogenic microbes and infectious particles in daily-used substances, like water, or in sanitary healthcare units, such as hospitals, health centers, public environments, and even indoors (10, 11). The amount of a disinfectant used to cleanse a surface or an area depends on the type of disinfectant and the desired level of disinfection; for example, imagine a healthcare-related zone, filled up with bacteria spores; an adequate amount of aldehydes can eliminate almost all spores, but lots of phenolic disinfectants, as they just affect the tridimensional structure of proteins, cannot remove any of them (12).
In the water treatment industry, disinfection byproducts (DBPs), like chloroform, bromate, bromodichloromethane, trichloroacetic acid, and formaldehyde, are usually formed through the reaction of chlorine, most frequently used disinfectant, with some poisons, carbon-based substances, iodide, and other similar species, which are totally named natural organic matter (NOM) (13-15). As the nanomaterials’ trace in pollution has been more scrutable, the role of carbon-based substances in the production of DBPs gets more obvious and could be considered as a remarkable issue in the modern nature (16).
Disinfection byproducts are commonly divided into two categories: hydrophobic and hydrophilic. The hydrophilic DBPs contain two major groups of chlorinated and non-chlorinated DBPs. The most common DBPs in drinking water are chlorinated DBPs. They are also divided into two classes: regulated and unregulated DBPs. Regulated DBPs are trihalomethanes (THMs), haloacetic acids (HAAs), and bromates. The major unregulated group of DBPs, on the other hand, includes chlorate and chlorite (13, 17-19). The most famous THMs are trichloromethane (commonly known as chloroform), bromodichloromethane, chlorodibromomethane, and tribromomethane (commonly known as bromoform). Haloacetic acids are a wider group with 3 subgroups, including monohaloacetic acids (monochloroacetic acid and monobromoacetic acid), dihaloacetic acids (dichloroacetic acid, dibromoacetic acid, and bromochloroacetic acid), and trihaloacetic acids (trichloroacetic acid, chlorodibromoaceti acid, bromodichloroacetic acid, and tribromoacetic acid). Trihalomethanes and HAAs are formed due to the reaction between chlorine and NOM in chlorinated water. Bromate and chlorite are also known as inorganic DBPs, which can be formed after water and wastewater disinfection by chlorine dioxide and ozone, particularly in water resources with high bromide content (20-22). Other halogenated DBPs are haloacetonitriles, cyanogen chloride, and mutagen X (MX). Also, the reaction of ozone with natural organic matter can lead to the formation of aldehydes, ketoacids, and carboxylic acids (5, 13, 17, 23-25).
Chemicals used as water and wastewater disinfectants are able to either destruct or completely inactivate microorganisms; so, they have an anti-life nature (26). As a result of this quiddity, they possibily can affect the healthy body cells. They make their influence on the fundamental mechanisms of the cells, and according to the pathology of cancer, a possible correlation between DBPs and cancer is not out of mind (27-29). The association between DBPs and various types of cancer has been previously demonstrated (5, 12, 30, 31). Also, the matter of DBPs in causing cancer has been studied previously (32-35). The role of the current review is to address the underlying mechanisms and discuss the most common cancers caused by the continuous exposure to DBPs. Furthermore, the effective treatment methods and mechanisms of DBPs removal in drinking water is provided.
2. Evidence Acquisition
During current narrative review, which was conducted between November 2017 and January 2019, the consequences of DBPs, which are led to cancer, were investigated. A comprehensive literature review of research databases, containing Medline, PubMed, Scopus, Embase, Google Scholar, Cochrane Library, Toxline, Pollution Abstracts, Water Resources Abstracts, and BIOSIS previews was performed, using the main keywords of “cancer”, “drinking water”, and “disinfection byproducts”. Up to the end of the time period of the study, the published manuscripts were considered. Also, for improving the precision, a manual searching among the references of gathered articles was performed. MeSH terms and free text words were also included, regarding the inquiry method of some databases. Of total 7420 gathered articles, 4300 articles were excluded due to inconformity with the subject. From the remaining 3120 articles, 2510 were excluded after checking the abstracts by the research team. After inspecting the full text of the remaining 610 articles, 103 eligible articles were selected. The qualified manuscripts were reviewed and appraised based on their relation to the subject; the impact of DBPs on various cancers, and the subject-related contents and data were analyzed by the research team. In all processes, articles were appraised by two members of the research team and the points of differences in opinion were referred to a third arbitrator. Also, the research team contacted with the authors to obtain additional information, if necessary. Among all cancer types, the most relevant types were chosen and the relationship between them and DBPs was considered.
3. Results
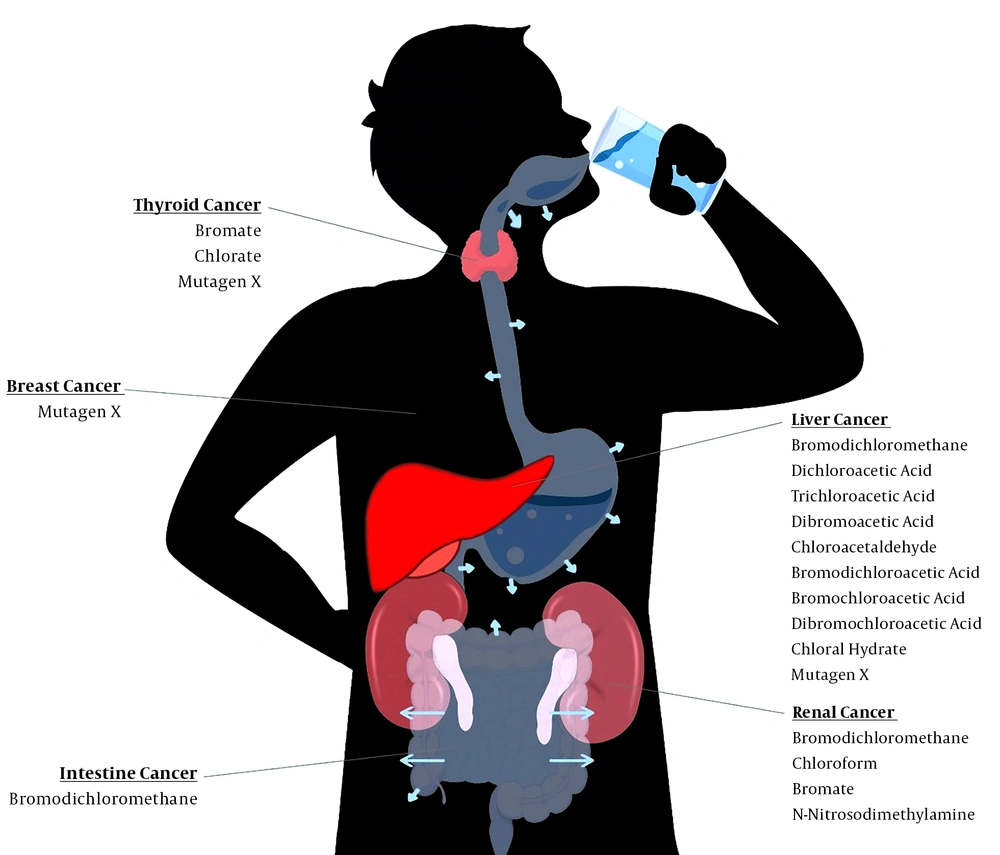
Disinfection byproducts can cause many different types of cancers (5, 13, 36), but some types are more likely to be developed due to exposure to DBPs. Previous studies showed that the most common cancers in laboratory animals, due to exposure to DBPs, are renal cancer, intestinal carcinoma, hepatocellular tumors, mesothelioma, and thyroid follicular cell tumor (13, 37). Some of them, like bromate, are probably human carcinogens too, meanwhile some are not known as human carcinogens yet (13, 38, 39). But, as the liver and kidney are the most common target organs for toxicity, they are most likely to develop tumors in humans (5). In a recently published systematic review with the aim of detecting the populations and regions vulnerable to bladder and colorectal cancer due to exposure of DBPs, the incidence of the mentioned cancers among different groups of people have been discussed; furthermore, it has been determined that socio-demographic characteristics may play an important role as risk factors (40-45). Most common cancers related to DBPs are discussed in the following paragraphs and summarized in Table 1. Figure 1 illustrates how DBPs pass from gastrointestinal tract into the circulation system and damage the vital organs. The number of arrows shows the absorption rate of different sites in Figure 1.
| Cancer Type | Known DBPs as Risk Factors | Year of Publication | Authors | Reference |
|---|---|---|---|---|
| Intestine cancer | Bromodichloromethane | 2010 | Rahman et al. | (32) |
| 2018 | Benmarhnia et al. | (40) | ||
| Renal cancer | Bromodichloromethane | 2014 | Burcham et al. | (5) |
| 2007 | Richardson et al. | (13) | ||
| 2004 | Komulainen et al. | (46) | ||
| Chloroform | 2014 | Burcham et al. | (5) | |
| 2007 | Richardson et al. | (13) | ||
| 2004 | Komulainen et al. | (46) | ||
| 2017 | Jone et al. | (47) | ||
| Bromate | 2014 | Burcham et al. | (5) | |
| 2006 | Moore et al. | (48) | ||
| N-Nitrosodimethylamine | 2002 | Choi et al. | (49) | |
| 2000 | Fujioka et al. | (50) | ||
| 2007 | Arinç et al. | (51) | ||
| Liver cancer | Bromodichloromethane | 2007 | Richardson et al. | (13) |
| 2017 | El-Halim et al. | (52) | ||
| Dichloroacetic acid | 2017 | Yang et al. | (53) | |
| 2000 | Lash et al. | (54) | ||
| 2004 | Pereira et al. | (55) | ||
| Trichloroacetic acid | 2017 | Yang et al. | (53) | |
| 2000 | Lash et al. | (54) | ||
| Dibromoacetic acid | 2007 | Melnick et al. | (56) | |
| Chloroacetaldehyde | 2007 | Richardson et al. | (13) | |
| Bromodichloroacetic acid | 2007 | Richardson et al. | (13) | |
| Bromochloroacetic acid | 2007 | Richardson et al. | (13) | |
| Dibromochloroacetic acid | 2007 | Richardson et al. | (13) | |
| Chloral hydrate | 2017 | Holmes et al. | (57) | |
| Mutagen X | 2007 | Richardson et al. | (13) | |
| 2017 | Holmes et al. | (57) | ||
| Leukemia | Dibromoacetic acid | 2007 | Program et al. | (58) |
| 2009 | Baccarelli et al. | (59) | ||
| Formaldehyde | 2010 | Zhang et al. | (60) | |
| Mutagen X | 2007 | Richardson et al. | (13) | |
| 2005 | McDonald et al. | (61) | ||
| 2009 | Zhang et al. | (62) | ||
| Thyroid cancer | Bromate | 2014 | Bull et al. | (63) |
| Chlorate | 2007 | Richardson et al. | (13) | |
| 2012 | Righi et al. | (64) | ||
| Mutagen X | 2017 | Holmes et al. | (57) | |
| 2012 | Righi et al. | (64) | ||
| Breast cancer | Mutagen X | 2007 | Richardson et al. | (13) |
| 2005 | Mcdonald et al. | (61) |
Abbreviation: DBPs, disinfection byproducts.
3.1. Intestine Cancer
Intestine cancer is one of the most common carcinomas all around the world (65). Intestine cancer could have numerous reasons, but the most involving mechanisms of occurrence contain the association between insulin-resistance and colonic adenoma, and epithelial barrier failure (66). The first mentioned mechanism can be a result of pancreas damage. On the other hand, most chemicals and toxins involve pancreas (5, 67). So, the chemicals, which hurt pancreas and involve its mechanisms, onset a cancerous intestine. The second mechanism, the damage of epithelial barrier, can also appear as a side-effect of exposure to chemicals. Trihalomethanes including bromodichloromethane, chlorodibromomethane, chlorodifluoromethane (CFC), chloroform, bromoform, and other similar compounds are the most common DBPs and top accusers of intestine cancer (32, 68-70). CFC is used as refrigerant and chloroform is also a useful solvent. According to previous researches, THMs can harm pancreas and cause intestine cancer by the first-mentioned mechanism (5, 71, 72). They can cause pancreas cancer, too. Also, the impact of THMs on the epithelial tissue of intestine is proven (52, 73). So, both predicted mechanisms are involved in the process of exposure to THMs and pathology of intestine cancer. Limiting smoking and alcohol consumption, exercising regularly, and lowering the dietary fat are effective ways to help reducing pancreas-damage and epithelial barrier failure risk (74, 75). It is necessary to mention that a multicenter case-control study conducted in 2016 disproved the association of lifetime total THM exposure and colorectal cancer, but regarding the high heterogenicity in the observed sample, more studies are needed to prove the point (76).
3.2. Renal Cancer
Kidneys carry the important role of preserving homeostasis and omitting blood wastes. Renal cancer makes up more than one out of every 30 cancers worldwide (77). Obesity, cigarette smoking, hypertension, renal failure, change of lining tissue, diabetes mellitus, trichloroethylene exposure, and consumption of analgesics are known risk factors for renal cancer (67, 78-80). As THMs (bromodichloromethane and chloroform) affect the epithelial and lining tissues of organs, renal cancer is a possible result of THM exposure (5, 13, 46). Bromate also increases the risk of kidney cancer by causing oxidative damage and inducing mutation to chromosomes in the kidney (5, 48). The mechanism of cancer occurrence by bromate may mostly lie on lipid peroxidation mechanism (81, 82). It is notable that lipid peroxidation increases in hypertension and obesity (81). So, both THMs and bromates have a positive effect on renal cancer occurrence. N-nitrosodimethylamine (NDMA) is another carcinogenic disinfection byproduct, which follows the mentioned mechanisms and exposure to NDMA leads to methylated bases forming in the genomes. The formation of O6-methylguanine as a result of NDMA N-demethylase enzyme activity could be responsible for the carcinogenicity of NDMA (49-51).
3.3. Liver Cancer
The liver plays the important role of detoxification in the human body and is the most exposed organ to toxins (27). Also, many chemicals can cause liver problems; for example, exposure to aflatoxin is a major risk factor for the pathology of liver cancer (65). Multiple DBPs can cause liver cancer, including THMs such as bromodichloromethane (13, 52), HAAs such as dichloroacetic acid and trichloroacetic acid (53, 83), and many unregulated DBPs (13). Chloroacetaldehyde, bromochloroacetic acid, bromodichloroacetic acid, dibromochloroacetic acid, dibromoacetic acid, chloral hydrate, and MX, are among those DBPs with a proven effect on increasing the risk of developing liver cancer (13, 54-57). Liver cancer due to exposure to chloroform and THMs is roughly related to cytotoxicity and cell multiplying in tissues (46). Haloacetic acids can cause harm by direct DNA damages and inhibiting glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity and, therefore, onset the carcinoma forming in livers (84). MX has a mutagenic nature and is responsible for near half of the mutagenic quiddity of chlorinated water (85). Ionization of DNA bases due to reductive feature of MX can cause DNA damage. Also, DNA adduction is another considered mechanism of mutation caused as a result of exposure to MX (61). Liver carcinomas due to exposure to dibromoacetic acid are supposed to be part of the phenotype-based selective growth of a cell-type (56). Hence, the liver is the main target of DBPs.
3.4. Leukemia
Apart from the types of leukemia, this type of cancer is broadly known as a lethal one (1). Alongside with hereditary factors, ionizing radiation is knowingly associated with leukemia, accompanying by many environmental risk factors, such as cigarette smoking and electromagnetic fields (EMFs) (86). As witnessed in rats, leukemia could be associated with dibromoacetic acid exposure, as it is known to be carcinogen due to the peroxisome proliferation effect and cytotoxicity (58, 59). Also, Formaldehyde is carcinogenetic risk factors for leukemia, which affects DNA repair pathways and DNA damage responses (60). The invasion of bone marrow hematopoietic and blood stem cells are considered other possible mechanisms of the cancer pathology due to formaldehyde exposure (62). MX could also be mentioned as a leukemia inducer. Although the specific mechanism of MX carcinogenicity is uncertain, for its reductive characteristic, the ionization of DNA bases is the most considered mechanism of developing cancer-leading mutations due to MX exposure (13, 61). Chronic myeloid leukemia (CML) is the most common developed type as a result of exposure to DBPs (87).
3.5. Thyroid Cancer
Thyroid cancer is one of the most common endocrine carcinomas (88). Obesity, diet, lifestyle, radiation, and environmental pollutants are the most well-known risk factors for thyroid cancer (89). Bromate has a positive effect on the formation of thyroid malignancies by inducing the formation of 8-oxodeoxyguanosine (8-oxoG) in DNA and its products (63). The impact of chlorate and MX on thyroid cancer have also been discussed in previous studies and confirmatory evidence have been found (13, 64). The imitation of endocrine hormones could be a possible reason for the toxicity and carcinogenicity of MX and other hormone-like DBPs (57).
3.6. Breast Cancer
Breast cancer is the most diagnosed malignancy in the American women (90). Obesity, hormonal therapies, and genetic factors are the most common causes of breast cancer in women, alongside the exposure to chemicals (91). MX is the only known DBP that acts in the way of inducing breast cancer, as many other DBPs are not studied yet. The mechanism involving MX carcinogenicity could lie on the ionization of DNA bases and its mutation-inducing ability. DNA adduction may also be considered, but the certain mechanism is not specified yet (13, 61). As the breast cancer has got many more important risk factors, the effects of DBPs on this malignancy has not been a priority for previous researchers.
3.7. Approaches to Remove DBPs from Drinking Water
The main strategy for DBPs control is preventing their formation through NOM, major DBPs precursors, removal before peroxidation, and disinfection processes in treatment plants. However, after occurrence, drinking water DBPs can be reduced through different ways. Best available technologies include two precise methods: enhanced coagulation and granular activated carbon, commonly with an empty bed contact of 10 minutes have been suggested for DBPs removal (92). Granular activated carbon acts in two ways and reduces both DBPs and DBP precursors (93). Also, powdered activated carbon plays a positive role in the control of natural organic maters and DBPs (94). Point-of-use carbon devices are known as other useful carbon-dependent tools to control taste and odor of chlorine and its detrimental byproducts (95). Using membrane filters is another point of use removal technique that was also suggested for DBPs removal; however, in order to have a high removal efficiency, regular replacement, and orderly maintenance are necessary (96). Another established way to reduce DBPs in drinking water is the omission of pre-chlorination and changing the chlorination point to intermediate or post-chlorination, especially in large water systems (17). In this way, the application of chlorine dioxide is useful for reducing the impact of pre-chlorination elimination on latter therapeutic processes, and also augments the disinfection effect (97). Advanced oxidation processes such as plasma and ferrate-based processes are also studied for DBPs removal in lab scale; however, due to numerous degradation byproducts that is formed in this processes, more studies are required before their application in full scale water treatment plants (18, 23).
Regarding the differences in chemical interactions, effects, and properties of various disinfectants, replacing chlorine with other alternatives such as chloramines, ozone, chlorine dioxide, and UV is an effective way to lower the levels of DBPs (17, 98-102).
4. Conclusions
Based on the results of previous studies, liver and kidney are the most common target organs for toxicity by DBPs, which are on their way to becoming a major health-related problem, as the usage of disinfectants is increasing day by day and more cases get to be exposed. Bromodichloromethane and MX have been known as the most affecting risk factors in cause of most of the cancers. It has been offered reasonable and argumentative evidence on the carcinogenetic nature of most DBPs, as the animal laboratory studies confirm this claim, too. By passing time and showing up the crisis of by-products accumulation, the urgency of this issue becomes clearer. Providing safe drinking water resources, limiting the unreasonable usage of disinfectants, using the membrane filters, changing the chlorination point, and alternating disinfectants with less harmful DBPs could be the joint pin of further actions in this field. Further studies should be conducted, as the effects of many DBPs are not studied yet, due to their apparently less importance.