1. Background
Cisplatin (CP); cis-(PtII(NH3)2Cl2) is known as an anticancer agent for treatment of solid tumors (1, 2). Nephrotoxicity is the most important side effect of CP (3) due to its accumulation in epithelial cells of tubules (4). The animals treated with single dose of CP showed an increase in the serum level of creatinine (Cr), blood urea nitrogen (BUN), kidney damage, and kidney weights (KW) (5-8). CP also causes inflammation, stress oxidative, and apoptosis in cells (9). Previously, we showed that CP induced nephrotoxicity is gender-related (2, 10, 11). The result of some animals’ experiments indicated that estradiol (Es) can intensify and testosterone (Ts) may improve CP induced nephrotoxicity (12, 13). To consider renal function, it is reported that CP reduced glomerular filtration rate (GFR) (14) and sodium excretion was different between male and female rats treated with CP (15). In addition, the renal clearance in animals treated with CP is also age-related (16). CP induced nephrotoxicity also related to platinum-based and its accumulation in the kidney (17, 18). The side effects of this drug also are dose-related (19). The single dose and the divided dose of CP therapy may alter the accumulation of CP in the kidney. Both single dose and the divided dose of CP therapy were considered in clinic for toxicity profile, and indicated the possible less kidney toxicity and more magnesium loss by single dose therapy (20); however, different results was found by others (21).
2. Objectives
Therefore, the high dose of CP treatment in divided manner may have different toxicity profile. Accordingly, we designed two protocols of CP therapy, and the effects of sex hormone on renal function markers after CP therapy were considered.
3. Methods
The male and female (180 - 250 g) Wistar rats (Animal center, Isfahan University of Medical Science, Isfahan, Iran) were used in this study. The animals were housed under conventional and controlled conditions (12/12 light/dark cycles; 23 - 25°C). All experiments were approved by the Ethics Committee of Isfahan University of Medical Sciences (IR.MUI.REC.1394.2.239).
3.1. Male Animals
The male rats were anesthetized with chloral hydrate injection (450 mg/kg; ip). A midline incision was made in the sub- abdominal region, and the epididymis and testis were pulled out and removed. One week later, they were randomly allocated to 6 experimental groups for 2 protocols of CP treatments; there were 3 groups in each protocol.
3.1.1. Protocol 1: Continues (Divided) Dose of CP Treatment
Group 1 (named vehicle, n = 6): The bilateral orchiectomized (OR) rats received sesame oil at the beginning of each week intramuscularly as vehicle for 3 weeks, and then sacrificed by the end of the 3rd week.
Group 2 (named CP, n = 4): The OR rats received the same regimen as group 1 except that CP (3 mg.kg/day, i.p) for 5 days was administrated during the 3rd week.
Group 3 (named Ts + CP, n = 6): The OR rats received Ts enanthate (10 mg/kg/week, IM, Aburaihan Co., Tehran, Iran) dissolved in sesame oil at the beginning of each week intramuscularly for 3 weeks in addition to CP for 5 days during the 3rd week, and then sacrificed by the end of 3rd week.
3.1.2. Protocol 2: Single doses of CP treatment
The protocol 2 included 3 groups of 4 (n = 5), 5 (n = 6), and 6 (n = 5). The only difference between these groups and the groups in protocol 1 (groups 1 - 3) was the dose of CP. The OR rats in protocol 2 received the same regimen as group 1, 2 and 3, but at the beginning of the 3rd week, they received a single dose of CP (7.5 mg.kg/day, i.p), and then sacrificed by the end of the 3rd week. The treatment dose of CP was based on our previous researches (5, 6).
3.2. Female Animals
The female rats were anesthetized with chloral hydrate injection (450 mg/kg; ip). After midline incision in the sub- abdominal region, the ovaries were removed. One week later and similar to male rats, they were randomly allocated to 6 experimental groups for 2 protocols of CP treatments; there were 3 groups in each protocol.
Groups 7 - 12 (n = 7, 6, 5, 6, 7, 6 respectively): The bilateral ovariectomized (OV) rats received the same regimen as group 1 to 6, respectively, except Es (250 µg/kg/week, IM, Aburaihan Co., Tehran, Iran) instead of Ts. The selected dose of Es was based on previous work (12).
All the animals were placed in metabolic cages for 6 hours before the end of experiment to collect urine output. Then, the blood samples were obtained, and the animals were sacrificed humanly. The kidneys were excised and weighted immediately. The left kidney was used for histopathology investigations via hematoxylin and eosin (H&E) staining. The renal damage was assigned as kidney tissue damage score (KTDS), and it was scored from 1 to 4, while zero score was recorded for normal tissue (7, 8). The right kidney was homogenized and centrifuged. The serum levels of creatinine (Cr) and blood urea nitrogen (BUN) and the urine level of Cr were determined, using quantitative diagnostic kits (pars Azmoon, Iran) by autoanalyzer (Technicon, Ireland LTD). The serum level of nitrite was determined by Griess method as described before (22). Briefly, the sulfanilamide solution was added to the samples and the mixture was incubated. Then, N-(1-naphtyl) ethylenediamine dihydrochloride solution was added, and the absorbance was determined at a wavelength of 540 nm. The nitrite concentration was calculated compared to the nitrite standard curve. The level of malondialdehyde (MDA) was measured manually (23). Briefly, 1 mL of 10% trichloroacetic acid (TCA) was added into 0.5 mL of the sample, and centrifuged at 2000 g for 10 minutes. Then 0.5 mL of the supernatant was mixed with 0.5 mL of 0.67% thiobarbituric acid (TBA), and after 10 min of incubating in the boiling water, the absorbance was determined at the wavelength of 532 nm after cooling.
3.3. Statistical Analysis
The data are presented as Mean ± SEM. The quantitative data were compared by one-way ANOVA, using LSD. The Mann-Whitney test was applied to compare the KTDS among the groups. P ≤ 0.05 was considered as statistically significant.
4. Results
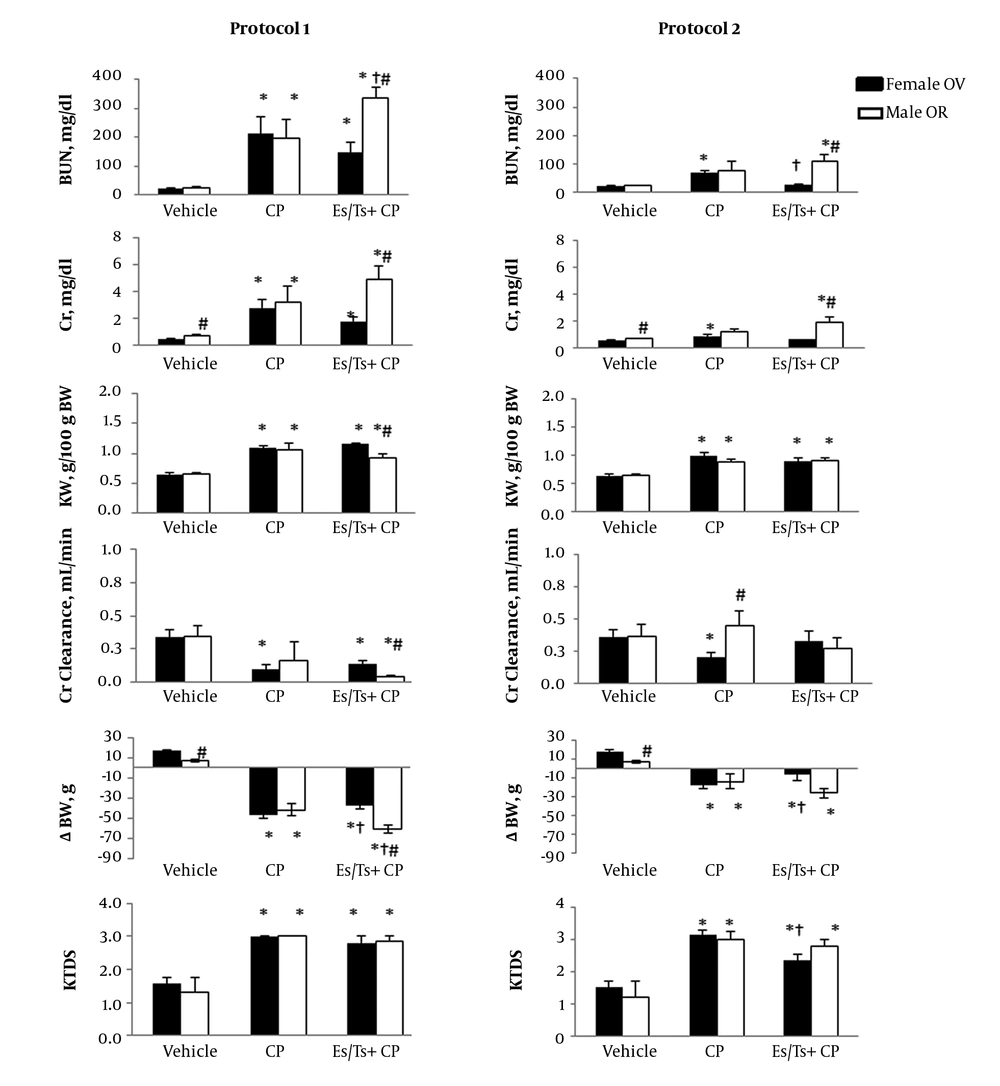
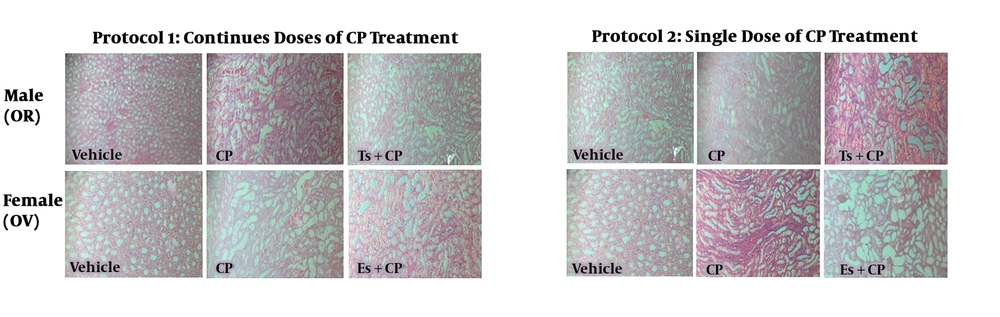
CP increased the serum levels of BUN and Cr in both male and female rats. However, Ts did not attenuate the levels of these markers toward normal values (Figure 1) in male rats, but Es in protocol 2 attenuated the serum levels of BUN and Cr. The KW and KTDS were increased and the body weight was decreased by CP, but Ts significantly increased body weight loss in protocol 1 compared with CP treated group (P < 0.05). Es did not attenuate KTDS in protocol 1 treated animals, but KTDS was decreased by Es in protocol 2 treated rats. Hormone therapy did not alter Cr clearance compared with CP treated group. The kidney nitrite and MDA levels altered differently in OR male and OV female rats (Table 1). In both protocols 1 and 2, the serum and kidney levels of nitrite were not significant between the groups in both genders. However, in female group from protocol 2 and male group from protocol 2, the kidney level of nitrite in vehicle treated group was significantly greater than the other groups (P < 0.05). In addition, the kidney levels of MDA in vehicle groups of male and female were significantly greater than other groups (P < 0.05). The samples images of kidney tissues are demonstrated in Figure 2. As observed, the CP induced tubular tissue damage was more than vehicle treated group in both male and female. The tubular damage was not reduced by hormone therapy (Figure 2).
| Treatment | Protocol 1 | Protocol 2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Female OV | Male OR | Female OV | Male OR | ||||||||||||
| Group | Vehicle | CP | Es/Ts + CP | P Value | Vehicle | CP | Es/Ts + CP | P Value | Vehicle | CP | Es/Ts + CP | P Value | Vehicle | CP | Es/Ts + CP | P Value |
| SNitrite, µmole/L | 5.8 ± 1.8 | 14.4 ± 5.7 | 4.2 ± 0.9 | 0.13 | 6.9 ± 1.3 | 8.3 ± 4.0 | 13.7 ± 3.7 | 0.26 | 4.1 ± 0.6 | 9.2 ± 2.3 | 6.6 ± 2.7 | 0.25 | 7.1 ± 1.5 | 3.9 ± 0.6 | 4.6 ± 0.5 | 0.08 |
| KNitrite, µmole/g tissue | 0.25 ± 0.02 | 0.16 ± 0.02a | 0.16 ± 0.04a | 0.008 | 0.24 ± 0.02 | 0.19 ± 0.04 | 0.20 ± 0.02 | 0.32 | 0.24 ± 0.02 | 0.21 ± 0.01 | 0.25 ± 0.02 | 0.28 | 0.23 ± 0.02 | 0.14 ± 0.01a,c | 0.18 ± 0.01a,c | 0.002 |
| SMDA, µmole/L | 4.3 ± 0.4 | 5.4 ± 0.6 | 5.6 ± 0.4 | 0.17 | 4.5 ± 0.7 | 5.0 ± 0.8 | 6.2 ± 0.9 | 0.33 | 4.5 ± 0.4b | 3.1 ± 0.4 | 5.0 ± 0.4b | 0.01 | 4.9 ± 0.7 | 5.5 ± 0.6 | 5.0 ± 0.7 | 0.8 |
| KMDA, nmole/g tissue | 14.5 ± 1.6 | 2.2 ± 0.7a | 0.81 ± 0.1a | < 0.001 | 13.2 ± 2.1 | 1.4 ± 0.6a | 1.9 ± 0.5a,c | < 0.001 | 14.5 ± 1.8b | 7.8 ± 0.9 | 12.2 ± 1.0b | 0.005 | 13.0 ± 2.6 | 6.1 ± 0.9a | 11.1 ± 1.5 | 0.05 |
Abbreviations: Es, estradiol; K, kidney; CP, cisplatin; MDA, malondyaldehyde; OR, orchiectomized; OV, ovariectomized; Ts, testosterone.
aRepresents significant difference from vehicle group in the same gender (P < 0.05).
bIndicates significant difference from CP group in the same gender (P < 0.05).
cShows significant difference between genders that receive similar treatment (P < 0.05).
The serum levels of blood nitrogen urea (BUN) and creatinine (Cr), and kidney weight (KW) per body weight (BW), Cr-clearance, BW changes (∆BW) and kidney tissue damage score (KTDS) in ovariectomized (OV) female and orchiectomized (OR) male rats received two protocols of cisplatin (CP) treatments (see text for protocols detail) with and without estradiol (Es) or testosterone (Ts). * Represents significant difference from vehicle group in the same gender (P < 0.05). † Indicates significant difference from CP group in the same gender (P < 0.05). # Shows significant difference between genders that receive similar treatment (P < 0.05).
5. Discussion
The major findings of this study reveal that Ts (in male) and Es (in female) therapy could not protect the kidney against CP induced nephrotoxicity. It is reported that male hormone testosterone synthesis is inhibited by administration of CP (24). Rostami et al. used different doses of Ts on a model of CP-induced nephrotoxicity in castrated male rats, and they demonstrated that low dose of Ts (10 mg/kg/wk for 4 weeks) decreased kidney tissue damage and the serum levels of BUN and Cr, while the high dose of Ts (100 mg/kg/wk for 4 weeks) did not protect the kidney against CP induced nephrotoxicity (13). The current results related to Ts (10 mg/kg/wk for 3 weeks) effect on the serum levels of BUN and Cr and KTDS were different from Rastami et al.’s findings (13), and the reason may be related to the duration of the hormone therapy. Accordingly, it seems that the duration of hormone therapy is an important factor to obtain a protective role against CP induced nephrotoxicity. The other study indicated that in renal ischemia/reperfusion in female gender, Ts inhibited the activation of nitric oxide synthases after ischemia; therefore, this hormone increased kidney susceptibility to ischemia (25). In addition, Ts promotes susceptibility of proximal tubule cells to apoptotic damage (26), while it may not have antioxidant properties (27).
The female hormone Es is well known as cardiovascular protectant agent in women before menopause. However, there is evidence that Es enhances oxidative stress in the kidney (28). Pezeshki et al. treated OV rats with single dose of CP accompanied with 3 different doses of estradiol, and they found that Es did not protect the kidney against CP induced nephrotoxicity (12). It is also reported that Es may abolish the nephron-protective effect of some antioxidants against CP induced nephrotoxicity (29). In agreement with those studies, the current finding did not indicate a nephro-protective role on renal function against CP induced nephrotoxicity. It seems that Es is a trigger to promote proximal tubules’ toxicity (30, 31), and even the antioxidant effect of Es (32, 33) did not attenuate the renal function disturbance induced by CP therapy.
5.1. Conclusions
It is concluded that 3 weeks of hormone therapy in animal model may not protect the kidney against CP induced nephrotoxicity. However, the renal toxicity intensity is related to the protocol of CP treatments, and single dose treatment may prefer, because its accumulation in the kidney is the main cause of renal toxicity (18).