1. Background
The rate of treatment in pediatric patients with acute lymphoblastic leukemia (ALL) depends on the region where they are treated (1). Recently 5-year overall survival rate improved to more than 90% worldwide (2). Literature reviews on Iranian pediatric patients with ALL reveal variant survival rates (3-6). In this regard, improving the outcome and prognosis of pediatric patients with ALL are the most important challenges for a physician. Treatment failures will impact on the overall survival of patients. Resistance to chemotherapy drugs is the main cause of treatment failures (7), and thus recurrence is the prominent challenge of those failures (8). Nearly 10% - 15% of pediatric patients with ALL will confer with relapse (9).
Different cellular mechanisms can lead to multidrug resistance (MDR), but one of the most important issues is the expression of ATP-Binding cassette transporters (10-12). ABCB1 gene (located on chromosome 7q21) is known as multidrug resistance gene 1 (MDR-1). Encoding P-glycoprotein (P-gp) can cause resistance to chemotherapy agents such as adriamycin (Adr), daunorubicin (DNR), paclitaxel, vincristine (VCR), and vinblastine (13-16). ABCG2 gene (located on chromosome 4q22.1) which encodes breast cancer resistance protein (BCRP) has an important role in resistance to mitoxantrone, methotrexate (MTX), cladribine, and topotecan (1). ABCG1 gene (located on chromosome 21q22.3) also known as ABC8 or WHITE1 (17) can make resistance to doxorubicin (18). Still published reports about multidrug resistance by ABCG1 in pediatric malignancies are rare.
2. Objectives
This study was designed because of two challengeable issues: (1) prominent rate of relapse in Iranian pediatric patients with ALL; (2) rare studies about the affection of ABCG1, ABCG2 and ABCB1genes’ expression on chemotherapy regimens of mentioned patients. The main objective of this study was whether the gene expression of ABCG1, ABCG2, and ABCB1 has a role in the recurrence of Iranian pediatric patients with ALL.
3. Methods
3.1. Patients
Enrolled children consisted of patients younger than 15 years old with the immune-phenotyping report of ALL who referred to an NGO referral childhood’s malignancy center (MAHAK Pediatric Cancer Treatment and Research Center) in Tehran, Iran. The study conducted case-control designing for evaluating gene expression of ABCG1, ABCG2, and ABCB1. Inclusion criteria for two groups were as: the case group consisted of mentioned patients who had relapse immediately or during their chemotherapy and at the time of the study were still administrating with chemotherapy regimens; the control group implied aforementioned patients who completed the treatment without any recurrence and at the time of study had 3 years relapse-free survival (RFS).
3.2. The Cumulative Dose of Chemotherapy Agents
The chemotherapy regimen of patients was based on the ALL-BFM (Berlin-Frankfurt-Munster) protocol 2009. Cumulative doses of administered chemotherapy agents in the induction phase were calculated. Cumulative dose of Vincristine (VCR) was categorized as low (< 20 mg/m2), intermediate (20 - 40 mg/m2), and high dose (> 40 mg/m2) (19, 20). According to the standard dose of daunorubicin (DNR) (21), the cumulative dose was categorized as standard (< 100 mg/m2) and high (≥ 100 mg/m2). The cumulative dose of L-Asparaginase (L-ASP) was categorized as standard (< 60000 u/m2) and high (≥ 60000 u/m2) doses.
3.3. Total RNA Isolation
Three milliliter of peripheral blood (PB) obtained from each child in the sthylenediaminetetraacetic acid (EDTA) blood sample tubes. Subsequently, the peripheral blood was immediately transferred to the laboratory. White blood cells were separated from PBs by Red Blood Cell Lysis Buffer (RBCL) and were suspended in Phosphate-buffered saline (PBS) (22). Total RNA was isolated by YTzol pure RNA reagent according to the instruction manual (cat No.: YT9063, Yekta Tajhiz Azma, Tehran, Iran). The concentration of total RNA was considered by Thermo Scientific NanoDrop (Thermo Fisher Scientific, USA) and the purity was checked by OD260/OD280 absorption. Total RNAs had been stored in -70ºC until cDNA synthetizing.
3.4. cDNA Synthesis
cDNA was prepared using the standard of total RNA. cDNA synthesis kit (Yekta Tajhiz) was used. The template RNA (based on 1 µg) was mixed with 1 µL random hexamer primer and finalized with DEPC (diethyl pyrocarbonate)-treated water to the volume of 13.4 µL. After incubation at 70ºC for 5 minutes, each product was mixed with 4 µL of 5× first strand buffer, 1 µL dNTP, 0.5 µL RNasin, and 1 µL of MMLV. The mixture was incubated for 1 hour at 37ºC and terminated by heating at 70ºC for 5 minutes. Finally, PCR products of synthesizing were stored in -70ºC for real-time PCR section.
3.5. Real-Time PCR (RT-PCR)
Primers for ABCG1, ABCG2, and ABCB1genes (23) were designed according to the Table 1. GAPDH gene was used for the normalization of the results through RT-PCR with a forward primer (5’ - 3’): GAAGGTGAAGGTCGGAGTC and reverse primer (5’ - 3’): GAAGATGGTGATGGGATTTC (24, 25). The specificity of primers had been checked with primer blast (26). All primers were obtained from Pishgam Biotech (Tehran, Iran). Primers had been purified with MOPCTM purification method.
| Gene | Forward Primer, 5’ - 3’ | Reverse Primer, 5’ - 3’ |
|---|---|---|
| ABCG1 | CCGACCGACGACACAGAGA | GCACGAGACACCCACAAACC |
| ABCG2 | CAGGTCTGTTGGTCAATCTCACA | TCCATATCGTGGAATGCTGAAG |
| ABCB1 | GTCCCAGGAGCCCATCCT | CCCGGCTGTTGTCTCCATA |
Before starting RT-PCR, products of cDNA synthesizes had been diluted as 1:3. For making a mixture of RT-PCR, 0.5 µL of forwarding primer + 0.5 µL of reverse primer + cDNA template according to 1 µg/µL + 7.5 µL of Syber Green QPCR master mix 2× added together and with nuclease-free water, the final reaction volume became 20 µL. The RT-PCR program had been set up as: one cycle of pre-incubation (95ºC = 300 s); 38 cycles of 3 steps amplification (95ºC = 60 s, 60ºC = 60 s, 72ºC = 30 s), and one cycle of melting (95ºC = 10 s, 65ºC = 60 s, 97ºC = 1 s). Quantifications had been done by LightCycler 96 real-time PCR Cycler (Hoffmann-La Roche AG, Basel, Switzerland). For checking the fluorescent signals of specific bands, all of the RT-PCR products were run by 1.5% agarose gel electrophoresis.
3.6. Statistical Analysis
Raw data were analyzed by light cycler software. Relative expression analysis was done by REST 2009 software. Statistical calculations were performed using SPSS version 22 software. Student’s t-test and chi-square were used for consideration differences between groups. P value was defined as ≤ 0.05 for significant relations. Pfaffl test was used for evaluating the relative expression of genes in two groups of case and control.
3.7. Informed Consent
Informed consent was obtained for peripheral blood sampling from enrolled patients. In this regard, all of the experiments were performed in compliance with the relevant laws. The Ethical Committee of Shahid Beheshti University of Medical Sciences approved this study.
4. Results
4.1. Patients
A total of 39 children with ALL were enrolled in this study. During 16 months, there were 23 patients for the case and 16 patients for the control groups.
The male to female ratio in this study was 1.3 (male = 22; female = 17). The mean age at diagnosis was 6.1 ± 4.1 years old (range 7 months to 14 years old). Immune-phenotypes of patients were as: Pre-B = 21; early Pre-B = 13; T-cell = 3; Pro-B = 2. Bone marrow aspiration (BMA) at the end of induction showed that 63% had complete remission, 29.6% as hypo-cellular and 7.4% as partial remission.
Sites of relapse in the patients in the case group were as: CNS = 55%; bone marrow = 25%; testis = 13%, and one patient had relapsed at bone marrow and CNS. The median time of relapse from diagnosis for those mentioned patients was 23 months (range 65 days to 5 years).
Table 2 shows patients’ characterizations according to the case and control groups.
| Male/Female | Mean age at DX ± SD, y | Immune-Phenotypes, N | BMA, % | VCR, % | DNR, % | LASP, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-B | Early Pre-B | T-cell | Pro-B | CR | HC | PR | LD | ID | HD | SD | HD | SD | HD | |||
| Case (n = 23) | 17/6 (2.8) | 5.1 ± 0.9 | 13 | 6 | 2 | 2 | 50 | 35.7 | 14.3 | 57.1 | 14.3 | 28.6 | 75 | 25 | 84.6 | 15.4 |
| Control (n = 16) | 5/11 (0.4) | 7.3 ± 1.0 | 8 | 7 | 1 | - | 76.9 | 23.1 | - | 13.3 | 26.7 | 60 | 90 | 10 | 73.3 | 26.7 |
Abbreviations: BMA, bone marrow aspiration; CR, complete remission; DNR, daunorubicin; DX, diagnosis; HC, hypo cellular; HD, high dose; ID, intermediate dose; LASP, L-asparginase LD, low dose; PR, partial remission; SD, standard dose; VCR, vincristine.
4.2. Patients’ Follow-Up
At the time of preparing this manuscript, 9 patients from the case group died because of their relapse. The median follow-up time for all of the patients was 4 years (range 14 months to 10 years). The median follow-up time for patients in the case and control group was 36 and 61 months, respectively. The 3-year overall survival and 5-yearoverall survival according to Kaplan-Meier analysis were: 82.3% ± 0.06 and 75.4% ± 0.07, respectively.
4.3. Cumulative Doses
The maximum and mean (± SD) cumulative dose of VCR were 90.9 mg/m2 and 38.5 ± 22 mg/m2, respectively (low dose: 34.5%, intermediate dose: 20.7%, and high dose: 44.8%). The maximum and mean (± SD) cumulative dose of DNR were 185 mg/m2 and 67.3 ± 43.5 mg/m2, respectively (standard dose: 81.8%, high dose: 18.2%). Based on the results the maximum and mean (± SD) cumulative dose of L-ASP were 92500 u/m2 and 43894.26 ± 22717.9 u/m2, respectively (standard dose: 78.6% and high dose: 21.4%). Table 2 shows cumulative doses of chemotherapy agents of the induction phase based on two considered groups.
4.4. Expression of ACG1, ABCG2, and ABCB1 by RT-PCR
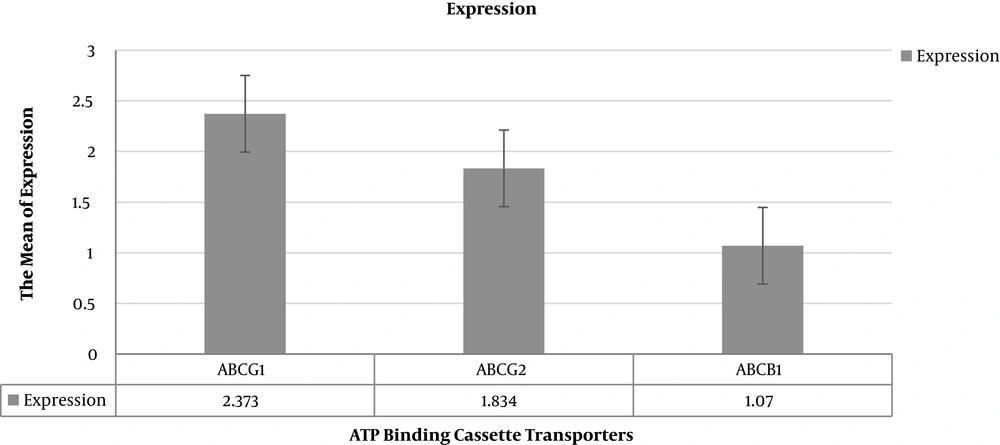
The reaction efficiency for GAPDH was 98%, while it was 96% for ABCG1, ABCG2, and ABCB1 genes (27). The relative expression analysis of ABC transporters’ genes is summarized in Table 3.
| Gene | Relative Expression | 95% Confidence Interval | P Value |
|---|---|---|---|
| ABCG1 | 2.373 | 0.157 - 36.607 | 0.0068 |
| ABCG2 | 1.834 | 0.140 - 22.248 | 0.0120 |
| ABCB1 | 1.07 | 0.069 - 19.605 | 0.0029 |
Patients with relapse had the significantly more relative expression of ABCG1 (P value = 0.0068), ABCG2 (P value = 0.0120) and less expression of ABCB1 (P value = 0.0029) in comparison to patients who had 3-year RFS. The relative fold of expressions in the case of the group according to Pfaffl test was summarized in Table 3. Figure 1 shows the relative expression of ABC transporters’ genes in the patients using REST 2009 software.
Analyses of paired t-test samples for considering the relation between means of ABCC transporters' gene expression and administrative cumulative dose of chemotherapy agents revealed significant relation (P values = 0.0001). High doses of VCR, DNR, and L-ASP led to high expression of ABCG1 and ABCG2.
5. Discussion
Multidrug resistance as a major issue through induction chemotherapy in pediatric patients with acute leukemia is still a prominent topic in cancer research (28, 29). Modifying in gene expression of ABC transporters can lead to MDR (30). In the present study, gene expression of ABCG1, ABCG2, and ABCB1were evaluated in pediatric patients with ALL. The resulted data approved that there was a high expression of ABCG1, ABCG2, and low expression of ABCB1 in patients who had relapsed ALL.
In 2017, Carrillo et al. devised a study on newly diagnosed patients with ALL to evaluate the expression of ABCB1 and ABCG2. Their results demonstrated high expression of mentioned genes in patients with ALL in comparison to healthy donors. Also, they suggested that early detection of ABCB1 could account as a risk factor diagnosis and following up of patients with ALL (1).
Rahgozar et al. (30) evaluated the expression of ABCB1 and ABCG2 in pediatric patients with ALL through two groups of new cases and relapsed ALL. Their results showed high expression of ABCG2 (1.35 ± 0.30) and low expression of ABCB1 in relapsed patients with ALL. According to those findings, they concluded that the expression of ABCG2 could be involved in the drug resistance of pediatric patients with ALL (30).
In another approach by Farawela et al. (31) gene expression of ABCB1 was evaluated in 37 Egyptian patients with ALL. Patients have consisted of new cases and individuals with complete remission, relapsed and resistant patients. Their findings revealed that there was a weak relation of gene expression of the aforementioned gene between new cases and relapsed ALL patients. Although ABCB1 had more expression in resistant patients (31).
There was a report that ABCB1 expresses nearly 13% - 40% in de novo cases with acute leukemia and 80% of patients with relapsed acute leukemia (31). In concordance with our findings, low expression of ABCB1 was also determined in some studies like in pediatric relapsed ALL patients by Lu et al. (32), or in young patients with relapsed ALL by Gurbuxani et al. (33), and finally the latest study by Farawela et al. who reported the low expression of ABCB1in patients with relapsed ALL (31).
ABCG2 gene encodes breast cancer resistant protein (BCRP) which plays a role in resistance to mitoxantrone, doxorubicin, and daunorubicin (34). Overexpression of this gene has been reported through variant studies. Jaramillo et al. published a paper (2019) about the relation of some ABC transporters’ gene expression and MTX resistant in children with ALL. They highlighted that considered patients with overexpression of ABCG2 were resistant to MTX. Their results approved the chemoresistance feature of ABCG2’s overexpression (29).
Ross et al. (35) and Benderra et al. (36) approved high expression of ABCG2 which related to prognosis and survival rates of patients with AML in their studies. Sauerbrey et al. (37) carried out another study with controversial results on patients with ALL. Their results revealed that there were not any differences in the gene expression of ABCG2 in two groups of relapsed and new cases patients with ALL (37).
Results of this study do not have concordance with Sauerbrey’s report. In this study expression of ABCG2 in relapsed ALL cases was high. Also, this high expression was related to high doses of administered VCR, L-ASP, and DNR.
Published studies about the role of ABCG1 in drug resistance are limited. In this regard, any information about multidrug resistance of this gene will be valuable. It is believed that the main role of ABCG1 is homeostasis of cholesterol in macrophages (38). Denisov et al. (39) considered the drug-resistant profile by some ABC transporter genes in patients with different morphological breast cancer. It was found that ABCG1 was expressed in all of the morphological forms of patients. They could only detect the expression in the level of protein by immunohistochemistry (39). Because of scarce data about this gene, there is not enough information if it expresses in hematologic malignancies or not.
In the present study, according to our information, it might be for the first time that gene expression of ABCG1 was evaluated in pediatric patients with ALL. The analysis showed a significant correlation with the overexpression of ABCG1 and recurrence of ALL. Due to this result, planning further studies about the multidrug resistance role of ABCG1 is an inevitable value.
5.1. Conclusions
The main idea of devising this study was to determine whether the expression of ABCG1, ABCG2, and ABCB1 gene could be identified as the prominent risk factor of relapse in pediatric patients with ALL. The achieved data demonstrated that the expression of ABCG1 and ABCG2 could be related to this hypothesis. Therefore the authors consisted of designing further cohort studies to evaluate the possible roles of these genes in prognosis and outcomes of pediatric patients with ALL. Impact of high drug expression notably ABCG1 and ABCG2 could be account as prominent factors for conferring relapse in pediatric patients with ALL.