1. Context
Oral cancer is one of the 10 most prevalent malignancies in the world. Only in the United States, an estimate of 34000 new cases are reported annually (1, 2). Among the oral cancers, oral squamous cell carcinoma (OSCC) is the most common, making up more than 90% of malignancies in this region (3). Despite recent advances in the treatment modalities, including surgery, chemotherapy, and radiotherapy, the mortality rate of OSCC (mainly due to its lymphatic involvement and metastasis) still shows an increasing trend, which poses a challenge toward both patients and healthcare systems (3-5).
It seems that a better understanding of the mechanism underlying cancer initiation and growth provides us with better therapeutic approaches against malignancies, as well as beneficial countermeasures to prevent them. In this study, we will focus on the more recently proposed concept of cancer stem cells (CSCs) in oral premalignant and malignant lesions, offering an insight into the stem cell markers, their putative role, and the means of targeting them in treatments.
2. Evidence Acquisition
To determine the role of cancerous and precancerous stem cells (pCSCs) in malignancies and also their markers in OSCC, a systematic search was conducted in 3 databases, including PubMed, ISI, and Scopus. Using the main keywords of “cancer stem cell”, “oral squamous cell carcinoma”, and “cancer stem cell marker”, a comprehensive search was done among several research databases. Approximately, 470 papers were found. The primary selection of studies was done after reading their titles and abstracts. Subsequently, the remaining papers were studied and selected based on their relevance to our topic and also their strength. It is worth noting that in this study, we mentioned the markers, which have been discussed more often.
3. Results
3.1. The Origin of Cancer: Clonal Evolution Model
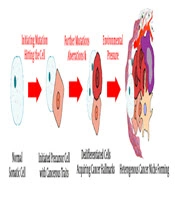
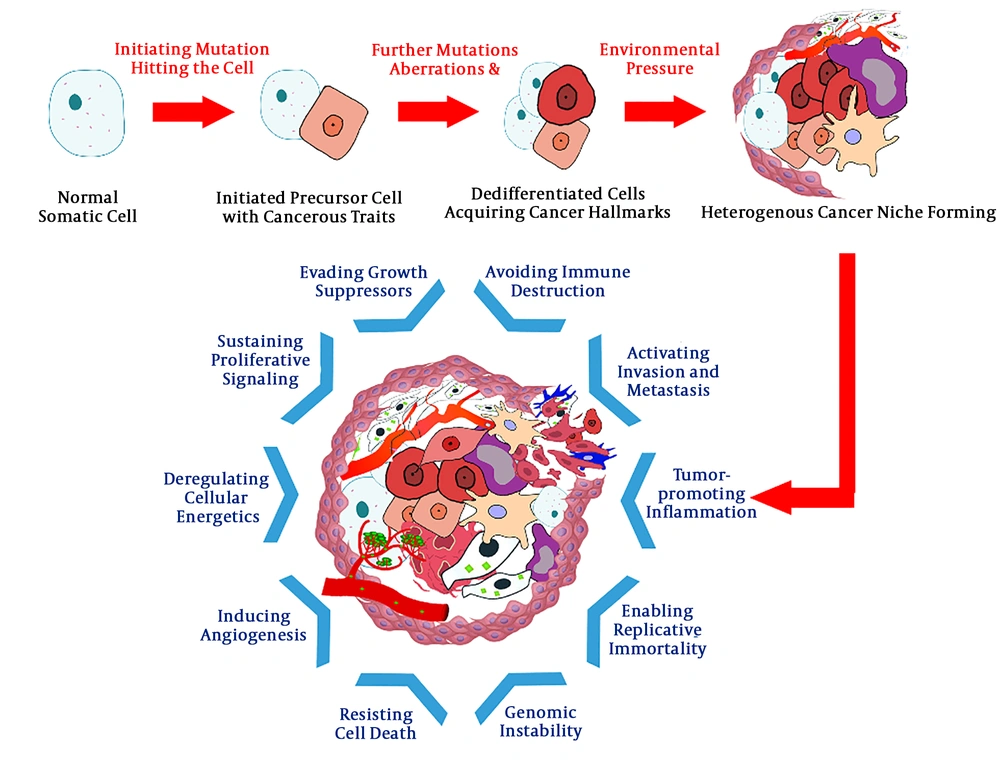
The efforts to reach a compelling answer to the mystery of cancers’ origin has a long history. Several theories have been proposed on the concept of carcinogenesis, among which the most popular is the “clonal evolution” model (6) (Figure 1). Based on this model, cancer is the consequence of multiple mutations striking the somatic cell’s gene consecutively. These mutations, interplaying with epigenetic aberrations, can alter the structure and function of normal regulatory genes; proto-oncogenes driving the cell’s proliferation, tumor suppressors inhibiting the cell’s growth, apoptosis-inducing genes, and genes involved in DNA repair. The result of this chain of nonlethal mutations is the formation of an “immortal” cell possessing a collection of traits, rendering an evolutionary competent cell lineage in the process of natural selection (7-10).
3.2. Cancer Stem Cell Model
An alternative model that is gradually gaining popularity is the “cancer stem cell” hypothesis. The idea that cancers arise from a distinct group of cells, known as “germ cells” or “stem cells”, was first proposed about 150 years ago (11). This concept became a hot topic again when Lapidot et al. tried to induce human acute myeloid leukemia in severe combined immunodeficiency (SCID) mice by transplanting different phenotypes of leukemic cell populations. They observed that only a rare population of cells that are less mature than colony-forming cells were able to initiate and propagate human AML in another host. These CD34+ CD38- cells were the first “stem cells” proved to be the origin of cancer (12).
In 2003, Al-Hajj et al. demonstrated that a small population of tumorigenic cells of CD44+ CD24-/low lineage was responsible for tumorigenesis in breast cancer (13). This was the first reported in vivo experiment in solid tumors, reiterating the role of a minor subset of cells with stemness characteristics in the formation and expansion of either blood-based or solid cancers.
Further studies and the inspection of intrinsic heterogeneities among cancer cells in a single tumor have led to the cancer stem cell hypothesis. The model, also known as hierarchical model, has 4 key concepts: (1) only a limited fraction of cancer cells have tumorigenic potential; (2) a distinctive profile of cell surface markers can be utilized to separate the CSC subpopulation from the rest of tumor; (3) the resulting tumor from CSC proliferation and differentiation consists of tumorigenic and non-tumorigenic cells, creating a heterogeneous environment; and (4) the CSC subpopulation can be serially transplanted through consecutive generations, suggesting its self-renewing capacity (14).
CSCs possess two principal features: the capability to regenerate the same stem cell (self-renewal) and to produce a progeny that can differentiate. Both traits are achieved by the CSC’s asymmetric division potential, as the daughter cell may follow either pathway of retaining the original identity or undergoing differentiation into various types of cells called “transit-amplifying (TA) cells”, which will eventually transform into the “more mature” cells. The stem cells may also undergo symmetric divisions, in which the mother cell divides into two similar daughter cells; maternally identical, resulting in CSC population expansion and tumorigenesis or both differentiating, resulting in the tumor bulk growth (15-17).
As a consequence of CSCs’ asymmetric division, a heterogeneous microenvironment consisting of different cells with different biological behavior is formed. It must be noticed that only CSCs and TA cells (cells with high proliferative capacity) can initiate and develop the tumor. Several alterations in the tumor microenvironment can form cells with other abilities such as migratory cancer stem cells (MCSCs), Radio-resistant cancer stem cells (RRCSCs), and chemo-resistant cancer stem cells (CRCSCs), which are responsible for cancer metastasis and relapse (15-17).
In addition, CSCs share several important properties with normal stem cells. The creation of CSCs is a multi-step process. Similar to the previously-explained clonal evolution, a cell has to “gain” some qualities and “lose” some other through several generations to ultimately turn into a cell with both “stemness” and “cancerous” traits- a cancer stem cell.
It is speculated that CSCs originate from 4 different cell types: (1) stem cells, (2) progenitor cells (PCs), (3) mature cells and more recently proposed, and (4) pCSCs.
3.2.1. Stem Cells
Human stem cells are unspecialized cells responsible for the formation and maintenance of tissues in the body. They are broadly categorized into 2 types: embryonic stem cells (ESCs) and adult stem cells (ASCs).
3.2.1.1. Embryonic Stem Cells
Three to 4 days after fertilization, a hollow ball of cells called the blastocyst develops, comprising an inner and outer layer. The cells in the inner layer are the ESCs. These pluripotent cells can give rise to all cell types of the body. ESCs are a transient class of cells, meaning that they require a special microenvironment and intercellular signaling to remain in an “undifferentiated” state. Thus, the ESCs cannot be normally found following the completion of body development (18).
3.2.1.2. Adult Stem Cells
During the entire lifetime, there are clusters of “organ-specific” resident stem cells in tissues called ASCs (15). The normal turnover of fully-formed tissues is dependent on the ASCs through proliferation and differentiation within their particular niches (19). Stem cells are long-lived with high self-renewal capacity and low-proliferation rates. In contrast, mature cells have a short lifespan, mostly do not self-renew, and proliferate more.
While ESCs have the potential to become every cell type in the body, ASCs are more likely to be “multipotent”, meaning they can only give rise to specific cell types of their tissue of origin (e.g. mesenchymal stem cells differentiate into connective tissues and hematopoietic stem cells into blood cells) (19). ASCs also express distinct cell markers allowing us to separate them from their tissue in addition to those “stemness” ones shared with ESCs.
There are several sites in the oral cavity with the identified populations of stem cells, including oral epithelium, connective tissue, and tooth structures (20). The above-mentioned properties also give stem cells exclusive durability against gene mutations, epigenetic changes, chemicals, radiations, and other death-inducing factors. All these bring up stem cells as the most expected source for CSCs and the initiation of precancerous and cancerous lesions in the oral mucosa.
3.2.2. Progenitor Cells
PCs are the “more specific” descendants of stem cells, meaning they are in a higher stage of differentiation. Compared with stem cells, a progenitor cell is more described as oligopotent (capable of differentiating into a few cell types) or even unipotent (only to a specific “target” cell type). The other difference between stem cells and PCs is that PCs cannot replicate indefinitely, and each division results in two differentiated cells.
Relative closeness to stem cells in terms of differentiation status and gene expression in addition to a higher number in tissues make PCs a good candidate for the accumulation of mutations and subsequent transformation into a cancer stem cell (21, 22).
3.2.3. Mature Cells
The theory that differentiated somatic cells can act as a source for cancers is somehow similar to the clonal evolution theory in concepts-typical somatic cells with a series of alterations that ultimately turn into a malignant cell (23).
As a part of normal body homeostasis, an injury to a region of a tissue triggers the stromal cells to release signals that induce surrounding cells to migrate to the wound site for healing. The role of parenchymal hepatic cells in the regeneration of the liver tissue following partial hepatectomy is evaluated (24). In another study, Takahashi et al. attempted to induce some genes (Oct-3/4, Sox2, c-Myc, and KLF4) in mature fibroblasts and drove them through a stemness status. These were the first cells termed as induced pluripotent stem cells (iPSC), the genetically reprogrammed mature cells capable of behaving like stem cells upon transplantation into the body (25).
What is more important to us now is the process, in which the differentiated epithelial cells act as a source for CSCs. In an interesting case, Radyk et al. studied mice with injuries to the lining of their stomachs. They found that even after blocking the signals that attract stem cells to the area, the epithelial cells undergo a metaplastic process toward a stem cell state, which can even lead to precancerous conditions in more chronic wounds. This is strong evidence for the role of mature epithelial cells in the development of stem cells with cancer-initiating properties (26).
Another phenomenon that can be utilized to vindicate the role of differentiated cells in cancer initiation is the epithelial-mesenchymal transition (EMT). Being a key role player in embryonic morphogenesis, EMT is mostly silenced in adult tissues (reactivated in pathologic conditions like wound healing, fibrosis, or cancer progression). During EMT, epithelial cells change in morphology, cellular structure, and gene expression and “switch” to a mesenchymal cell, giving them the ability to detach from nearby cells and migrate to their destination (27, 28).
It is clear that one of the hallmarks of every cancer is invasion and metastasis. This is achieved in a way rather similar to the EMT process. It is already shown that EMT might be involved in the creation of specific CSCs (i.e. EMT cancer stem cells) within tumor bulk that drive tumor invasion and metastasis (27, 29, 30). It is worth mentioning that the evidence is not strong enough yet to support the correlation between EMT and tumor metastasis in oral carcinomas (28). Additionally, the role of aberrant activation of EMT in gastric cancer initiation, as well as its progression and association with CSC theory, has been proven (26, 28). Given all these facts, it is quite reasonable to deduce that the same thing may happen in the outset of oral cancers, e.g. OSCC (31).
3.2.4. Precancerous Stem Cells
The idea that CSCs may arise from pCSCs is a relatively new one. The first evidence came out in 2003 when Gao et al. found a distinct subpopulation of cells in dendritic cell-like leukemic mice (32). These cells neither expressed hematopoietic and lineage nor hematopoietic stem cell markers. Interestingly, it was observed that these cells have the ability to transform into both benign and malignant lesions depending on the environmental condition. Based on this ability, the term pCSCs was coined (33).
Like CSCs, pCSCs can also originate from normal stem cells, PCs, and adult cells and possess the ability to self-renew and differentiate into more mature cell lines (17). They also express embryonic and adult stemness markers such as CD133, aldehyde dehydrogenase 1, and OCT-4, which can be utilized to isolate them from mature cells. Moreover, they can hide in the lesions’ microenvironment (17).
As mentioned before, depending on the microenvironment, these cells may give rise to primary CSCs and initiate a malignant condition. Several genetic and epigenetic factors contribute to this long process, making pCSCs distinct from CSCs. Chen et al. observed that during the transformation of pCSCs into CSCs in lymphoma induced in mice, lineage markers and CD45 were upregulated. These were in association with the expression of CD117 and SCA-1, the two markers that indicate the progression of different types of cancer. They also found that PIWIL2 (a PIWI/AGO family gene expressed in ESCs) can promote the proliferation of pCSCs (32, 33). A study in 2008, which compared the gene expression pattern between ductal carcinoma in situ (DCIS) (a premalignant condition) and invasive ductal carcinoma, found a difference in the expression pattern of 147 genes in these two lesions. Moreover, two genes, SULF1 and LOX, seemed to be correlated with the aggressive behavior of the tumor and can act as biomarkers to predict the risk of DCIS progression (34).
Therefore, we can differentiate CSCs from pCSCs according to the following criteria: firstly, precancerous cells can start either a benign or a malignant lesion based on the microenvironment condition while CSCs are responsible for the initiation and development of a malignant lesion. Secondly, pCSCs are found in precancerous lesions such as oral leukoplakia while CSCs are found in cancerous foci. The third is the epigenetic and genetic profile of CSCs and pCSCs (17). Taken together, it seems that the identification of pCSCs in precancerous lesions can provide us with the ability to evaluate the risk of malignant transformation of precancerous lesions and prevent their progression in the early stages.
3.3. Premalignant and Malignant CSC Markers
The progression of normal mucosa to mild, moderate, and severe dysplasia and then to an oral SCC is a multivariate process, comprising structural and functional changes in cells. The identification of the events relevant to malignant transformation is of the utmost importance. It can be useful for the clinician to evaluate the progression risk of premalignant lesions toward cancer, and preventive strategies can be performed. CSCs have been identified by using specific markers in various studies. Identifying a reliable CSC marker that is associated with cancer treatment is important (11, 35).
3.3.1. ALDH1
ALDH1 is an isoform of the ALDH enzyme family, serving as a detoxifying enzyme that oxidized aldehydes. It can also oxidize retinol (vitamin A) to retinoic acid (RA), the functional form of this vitamin (36). The overexpression of ALDH1 has been detected in the lower epithelial strata of oral premalignant lesions and has been correlated with the degree of cellular dysplasia (37, 38). Lesions with the higher expression of ALDH1 had a higher risk of transforming into a primary malignant lesion (38-40).
The presence of ALDH+ cells is significantly correlated with the histopathologic differentiation of the malignant tumor (41-43). Interestingly, although ALDH1+ cells are scattered in the OSCC microenvironment, they are not found in the areas adjacent to the keratin pearls (44). High ALDH expression has also been linked with a decreased overall 5-year survival of patients (43, 44). It seems that high ALDH+ expressing cells are more chemo- and radio-resistant (41, 45). One reason for this might be that cells with the higher expression of ALDH1 are more capable of metabolizing chemotherapeutic agents and free radicals produced following radiotherapy (46, 47). It is also speculated that the high expression of ALDH1 enables the migratory of cells to undergo EMT and, therefore, reside in the other tissues and spread the malignancy to the other organs (38, 42, 48, 49).
3.3.2. CD44
The CD44 antigen is a transmembrane glycoprotein encoded by the CD44 gene. Alternative splicing in the process of CD44 gene expression results in a large family of protein isoforms widely distributed on the surface of many cell types, functioning as receptors for various ligands, such as hyaluronic acid, collagen, matrix metalloproteinases (MMPs), and homing chemokines (36, 50). This makes CD44 involved in a series of cell functions, including tissue remodeling, matrix degradation, and cell migration, which also happens in a tumor’s growth, invasion, or metastasis (51).
CD44 is abundantly expressed on normal cells in head and neck tissues. To date, lots of work has been done on CD44 isoforms as putative biomarkers for CSC differentiation and prognostic implications (52). A study on the expression of CD44s (standard isoform) and CD44v6 (a CD44 splice variant) in normal mucosa, oral leukoplakia, and OSCC indicated that with the progression of normal epithelia toward dysplasia, the staining intensity slightly increases and extends to more suprabasal layers. CD44 expression in OSCC samples even showed a diminished rate (53). Another study on actinic cheilitis demonstrated that while the expression increases both in intensity and extent with dysplastic severity, cell distinction using CD44 is not easily possible in different tissue conditions (54). Several studies report that different CD44 variants are associated with higher grades, higher drug resistance, and poor prognosis in head and neck SCC (52, 55-57).
Generally, we can conclude that alteration in CD44 expression is a valuable factor for the early detection and prognosis of oral epithelial dysplasia and OSCC, although it may not be specific enough to determine cell types solely and other biomarkers are also needed to isolate CSC populations for further target therapies (47, 58, 59).
3.3.3. BMI1
B cell-specific Moloney murine leukemia virus integration site 1 protein (BMI1) is a member of polycomb group proteins encoded by the BMI1 gene. This protein acts by the remodeling of chromatins and modification of histones and serves a crucial role in the cell cycle (60). It is also believed that these genes are important in the maintenance and self-renewal property of embryonic and adult stem cells (60-62). The unregulated expression of these genes has been associated with many solid malignancies including HNSCCs (11). BMI1 expression is significantly higher in oral leukoplakia and OSCC compared to the normal oral mucosa and there is also a significant difference in the expression pattern of BMI1 in mild dysplastic lesions compared to moderate and severe ones (63). BMI1+ leukoplakias have a higher risk of transformation into a primary tumor (64).
The role of BMI1 in HNSCC seems to be a matter in dispute, asking for further studies. However, we believe that BMI1 upregulation is significantly connected with the invasive properties of the tumor and also the overall survival of patients (63, 65-67). The higher expression of BMI1 has been observed in invasive cells, which was accompanied by the upregulation of vimentin and downregulation of E-cadherin, linking this marker to the EMT process (65, 68, 69). BMI1+ cells are also believed to resist chemotherapeutic agents (69, 70). This might be due to the high expression of “AP-1”, a transcription factor linked to tumor metastasis and chemoresistance. The inhibition of BMI1 or AP-1 has reduced the resistance of HNSCC cell lines in animal models (71).
The studies by Tamatani et al. (44) and Hayry et al. (72) showed a negative connection between BMI1 upregulation and tumor invasion, as well as overall survival rate. This can be due to the fact that these studies only investigated patients in the early stages of their disease. Also, some studies included patients in early and advanced stages and suggested no significant association between BMI upregulation and the overall survival rate (73). However, their study populations were much lower than other similar investigations. Therefore, it seems that more concise studies with sufficient target populations are still required to shed light on this topic.
3.3.4. p75NTR
The p75 neurotrophin receptor (p75NTR), also known as nerve growth factor receptor (NGFR) or CD271, is a member of the tumor necrosis factor (TNF) superfamily. Based on the specific cell and ligands binding to it, p75NTR regulates various cellular activities like cell growth, mitosis, or apoptosis through different signaling pathways (74, 75). CD271 is found basically on neurons, and it was later found to be a marker for stem cells (76). More recently, it has been proposed as a putative CSC marker in the oral dysplastic and malignant epithelium (mostly in conjunction with other markers such as CD44 and ALDH1) (40, 54). Some studies on the expression of p75NTR have shown that its staining pattern does not differ much between normal oral mucosa and dysplastic lesions, being confined to the basal layer (40, 77). In the case of OSCCs, the staining extends from the cellular nests’ margins in lower grades to the inner layers in higher grades (78). Another study indicated that stain intensity increases with the progression of oral dysplasia’s severity. Given its good specificity in the determination of tumor-initiating cells (TIC) clusters in the oral SCC tissues. Murillo-Sauca et al. (79) and Tong et al. (80) have shown that CD271 is a useful marker for identifying and targeting the biologically active cancerous cells.
3.3.5. CD133
CD133, OR Prominin 1, is a pentaspan transmembrane glycoprotein that is expressed in adult and embryonic epithelial cells and also non-epithelial cells such as hematopoietic stem cells (81). This protein is widely used as a CSC marker in many solid malignancies (82, 83). Liu et al. found that CD133+ in patients with oral leukoplakia had a 2.86 fold chance to transform into oral cancer compared with CD133- cells (37). Moreover, a gradual increase of CD133 expression has been witnessed from normal to dysplastic and OSCC cells. The expression of CD133 was also significantly higher in the advanced stages of the OSCC compared to the early stages (84-86). An in vitro studied showed that silencing CD133 gene enhances the chemosensitivity of the OSCC side-population cells (87). The role of CD133 in metastasis remains contradictory and it seems we need more evidence to understand its role in the process of metastasis thoroughly (85, 86, 88, 89). It is necessary to note that we still lack enough evidence to understand the role of CD133 in different stages of OSCC development and to utilize this marker clinically.
4. Conclusions
The identification of the mechanisms underlying oral cancer initiation and progression is of the utmost importance. Despite advances in therapeutic options for OSCC over the last decades, mortality and morbidity rates have not been markedly improved. Therefore, the search for new and better CSC markers that relate comprehensively with the known alterations of tumor progression seems to be necessary. CSC markers that could act as a therapeutic target could play an important role in the effective treatment strategies of OSCC.