1. Background
Addiction is a gradual process that begins with occasional use and progresses to regular consumption, eventually leading to uncontrolled substance abuse. The main problem facing the practitioners is the high rate of return among abusers with an unsuccessful attempt to stop substance consumption. Substance abuse is a worldwide phenomenon that has caused the most severe damage to human societies. It is an uncompromising model of substance abuse that can cause acute symptoms, including a set of cognitive, behavioral, and psychological symptoms (1). Approximately 200 million people aged 15 - 64 worldwide take an illicit substance at least once a year (2). Iran, in this regard, has a large number of addicted people to substances due to its geographical characteristics and its borders with drug-producing countries (1). The last report of Iran’s drug control headquarter indicates the presence of 1.2 - 2 million consumers in the country. Furthermore, many individuals are either addicts or have at least someone addicted in their families (2).
The accumbens (AC) nucleus is a part of several groups in subcortical nuclei called the basal ganglia (basal nuclei). It plays a vital role in the return to substance use, and its stimulation produces promising results in the treatment of substance dependence. The AC sends efferent to areas such as the frontal cortex, limbic areas, amygdala, hypothalamus, and midbrain, which greatly contribute to the processes involved in reward. Studies have shown that addiction can decrease the activity of areas such as the cingulate cortex and dorsolateral prefrontal cortex (DLPFC), which, in turn, decreases a person's control over his behavior and ultimately leads to addiction. On the other hand, the AC receives important inputs from the prefrontal cortex, which affects the activity of the AC region and the addictive behaviors of the individual. One of the target areas studied when investigating DBS and addiction is the prefrontal cortex – the medial prefrontal cortex (mPFC), in particular (3). A behavioral study has evaluated the effect of high and low-frequency stimulation of mPFC (4) on dependency manners.
To date, no approved drug treatment has been developed for stimulant substance abuse. However, a new class of neurosurgery interventions has become popular to treat movement disorders and mood disorders. One of these prominent therapies is deep brain stimulation (DBS) (5). Electrical current, typically at frequencies above 100 Hz, is directed through electrodes implanted surgically in the subcortical brain nuclei (6). Preclinical studies and case studies have reported the therapeutic effects of DBS on addiction. Recent findings about the neural pathways affected by addiction have created a new range of possibilities for the treatment of addiction by targeting the involved neural pathways and returning their activity to normal status. There is a need for new effective interventions for patients who do not benefit from conventional treatments, since addiction is a chronic brain disease that seriously affects individual and social health (7).
In this respect, DBS research has explored various areas of the brain to treat neuropsychiatric conditions such as Alzheimer's disease (8), Tourette's syndrome (9), obsessive-compulsive disorder (10), and advanced depression (11). According to recent studies on animals and humans, DBS may serve as an effective treatment for addiction. Investigating the effects of DBS on the responses to alcohol, cocaine, heroin, morphine, and nicotine has produced encouraging results in several areas of the reward system (5). The effects of DBS are much more complex than simple local stimulation or inhibition. Instead, DBS has been shown to stimulate axonal terminals, exerting significant effects on neural circuits. However, the precise mechanism of DBS action has remained unknown. The impact of DBS depends on several parameters, such as the stimulated structure, its specific afferents and efferents, cell type, the ratio of primary neurons to interstitial neurons, mediating systems, etc. (12). In addition, different brain structures have different conductivities, and this parameter can fluctuate as a result of neurophysiological changes in different neurological disease states for which DBS is already used (13). However, DBS is a costly treatment for addicted patients that restricts access to available therapies (14). The side effects of DBS depend on the targeted locations and its application, and include speech disorder, memory disorder, aggression, mild mania, sexual hyperactivity, depression, and increased risk of suicide; furthermore, it can change the main aspects of the patient's personality and behavior (15). Insertion of stimulating electrodes can also lead to severe infections (14).
2. Objectives
To our knowledge, there was little known about the effect of DBS in mPFC on morphine dependency and motor activity. Therefore, the present study aimed to investigate the effects of high-frequency DBS (HF-DBS) in mPFC on the electrical response of neurons in the AC nucleus and dependency in morphine-addicted male rats.
3. Materials and Methods
Wistar adult male rats (200 – 250 g) were housed in standard plastic cages in a controlled colony room with standard conditions (temperature 21 ± 3°C, and 12 h light/dark cycle (lights on at 07:00 a.m.)) and with free access to food and water. The experiments were performed during the light cycle, and all animals were tested once. All experiments were performed based on the Guide for the Care and Use of Laboratory Animals (IR.ZAUMS.REC.1397.292).
3.1. Experimental Groups and Design
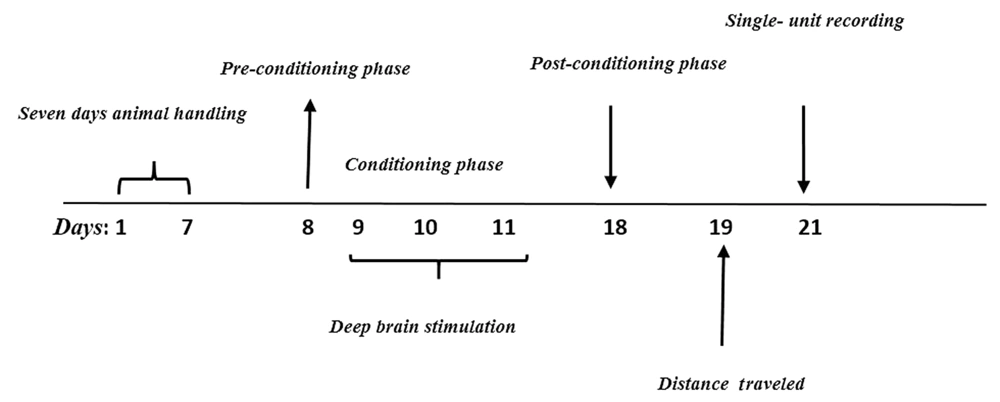
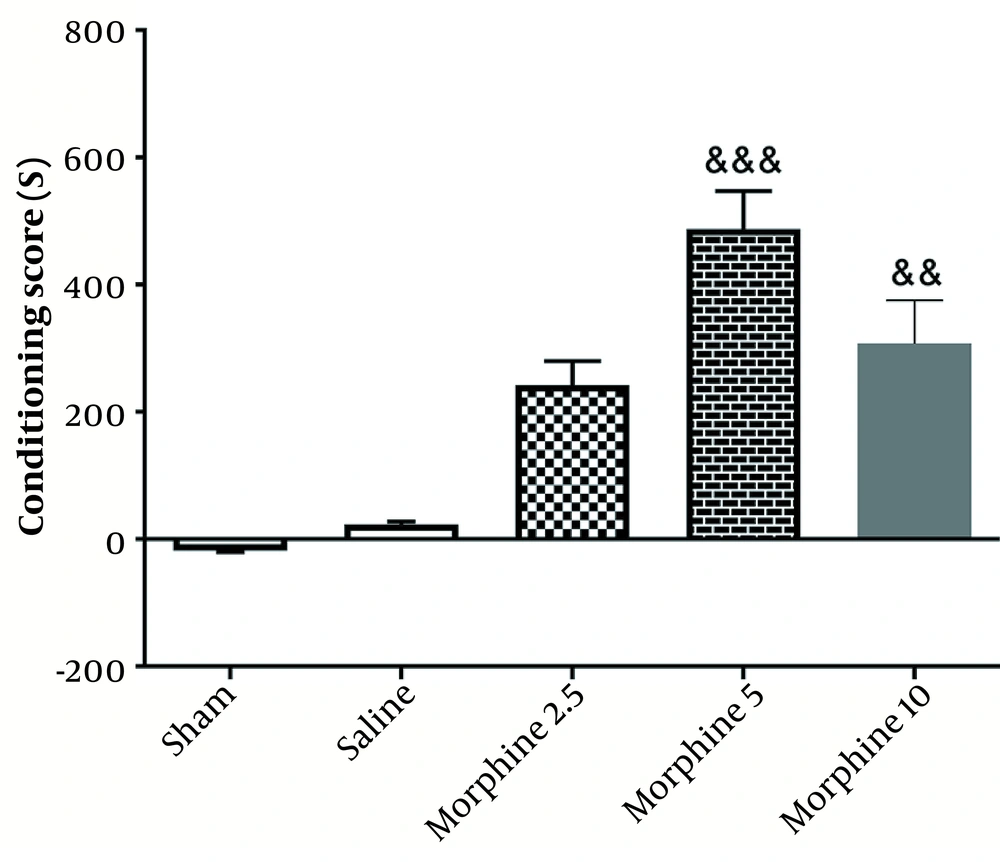
As illustrated in the experimental design (Figure 1), a pilot study was conducted to determine the optimum dose of Morphine. To this end, different doses of morphine (2.5, 5, and 10 mg/kg) were injected intraperitoneally. According to the results obtained from the conditioned place preference (CPP) test, the dose of 5 mg/kg was selected as the optimum dose (Figure 2).
Then, animals (n = 40) were assigned to five groups (n = 8), including saline, sham (animals were operated and a cannula was implanted), morphine (IP injection), saline+DBS, and morphine+DBS. All animals were used in withdrawal relapse, and extinction tests.
Animals were treated with saline or morphine according to the assigned groups. Then, the rats were subjected to stereotaxic surgery to insert the stimulator electrode in the mPFC and the recorder electrode in the AC. The electrical response of AC neurons to DBS was determined by adopting the single unit recording method.
The rats were anesthetized with an intraperitoneal injection of urethane (1.2 g/kg) during the procedure. An additional dose of urethane (0.1 g/kg) was injected to maintain deep anesthesia at one-hour intervals. Body temperature was maintained between 35.5 - 36.8°C by using a heating pad. The correct positions of mPFC and AC were then identified, and the skulls of the animals were perforated by a drill after considering the exact identified areas. The spatial location of AC was recognized based on the Paxinos atlas (AP: -4 mm, ML: ± 1.6 mm, and –DV: 8.4).
Extracellular recording of AC single neurons was performed by inserting a tungsten microelectrode (Harvard Apparatus, USA; Parylene Coated; 127 mm diameter; 5 MΩ) into the brain and using a stereotaxic device. Spike signals were amplified with a differential amplifier, and the produced sound was displayed on a monitor. Only neurons were included in the study, which had spike signals with stable amplitude and form. The action potentials were separated from the background activity using a window discriminator, which counted the number of spikes per unit time in addition to generating output pulses for the single-units based on the signal amplitude. Electrophysiological Data Acquisition was utilized to obtain samples from extracellular activity, and the obtained data were stored on a hard disk. Finally, single-unit activity was calculated as the frequency of spikes.
3.2. Conditioned Place Preference Test
To this end, a wooden device with dimensions of 30[L]x16[W]x45[H] and consisting of two chambers (i.e., a chamber with vertical lines and another chamber with horizontal lines) was used. The chambers were connected by a valve. This test was performed in three phases, including pre-conditioning, conditioning, and post-conditioning.
3.2.1. Pre-conditioning Phase
Each rat was separately located in the apparatus for 10 min on the 1st day. During this phase, the animal had free access to all chambers. Then, the presentation duration of the animal in each chamber was recorded and analyzed. Preference chamber for an animal was considered more tendency of the animal to one chamber.
3.2.2. Conditioning Phase
This phase lasted three days in conditioning sessions. The conditioning train was implemented on days 2 - 4, twice a day. All animals received 30-min sessions of saline and morphine for three days in the chamber, which was contrasted to the preferred chamber. Saline or morphine was injected in the morning-afternoon design alternately, with an interval of six hours. At the end of this phase, the rats experienced six times the effects of morphine and saline collectively. Access to another chamber was prevented on these days.
3.2.3. Post-conditioning Stage (Test Stage)
This phase was conducted on the 5th day (test day), in which the separator valve was removed, and the animals had access to the entire apparatus. The time spent in both parts of the apparatus for each animal was recorded for 10 min. The difference between the times spent in the drug-received chamber and the saline-received chamber was considered as the preference criteria to calculate the conditioning score. No injection was administered on the test day. Moreover, locomotor activity for each animal in all experimental groups was determined as a total traveled pathway for a 10 min period on pre- and post-days.
3.3. DBS Pattern
When a responsive neuron was identified, 5 min of baseline activity was recorded. During conditioning in the CPP box, the rats received DBS with a frequency of 130 Hz, the amplitude of 0.2 to 0.5 mA, and repeated periods of 15 minutes with an interval of 45 minutes for 3 hours. In this regard, a stimulator with proper programming potential was applied to achieve the mentioned stimulation pattern. DBS was performed by directing an electrical current from the stimulator to an electrode implanted in the rat’s brain. The stainless-steel electrode used in this study was bipolar and coated with insulating material. When a responsive neuron was identified, 5 min of baseline activity was recorded.
3.4. Locomotor Activity
Open field test (OFT) was performed to evaluate the motor activity of the rats. The OFT (80 cm × 80 cm × 50 cm) has a black body color with white lines. Every rat was placed in the center of the open field, and the number of lines crossed was recorded for 5 min (16). The field was cleaned between each trial by using 90% ethanol to remove odor cues. This field was divided into 25 squares of the same size, including a central area and 24 side squares, using Maze software.
3.5. Data Analysis
Data analysis and figure drawing were performed by using GraphPad Prism 8.1. The normality of data distribution was assessed by performing the d’Agostino–Pearson omnibus normality test. Behavioral and electrophysiological data were evaluated using One-way analysis of variance (ANOVA) and Tukey as a post hoc test. All data were presented as mean ± SEM, and the significance level was set at P ≤ 0.05.
4. Results
4.1. The Effect of Morphine and DBS on Conditioning Scores in Different Groups
As shown in Figure 2, chronic injection of morphine in 5 (P < 0.001) and 10 mg (P < 0.01) doses increased the conditioning score, which was indicative of CPP induction. In other words, the injection of morphine (5 mg/kg) significantly enhanced CPP [487.5 ± 59.91] [(F4, 25=37.5; P < 0.001)] in comparison with the sham group [18.33 ± 1.56].
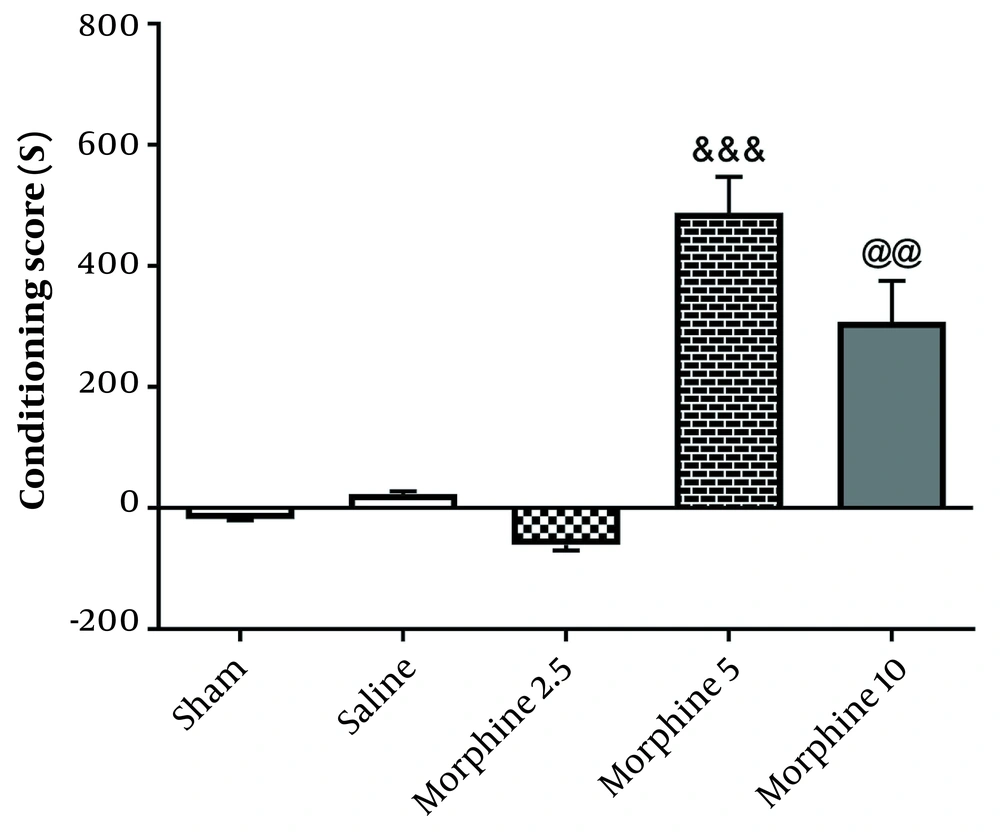
According to Figure 3, administration of morphine [487.5 ± 59.91] [(F4, 25=63.7; P < 0.001)] without electrical stimulation increased CPP scores compared with the sham group [18.33 ± 1.56; P < 0.001], but exposure to 130-Hz high-frequency morphine+DBS [308 ± 27.58; P < 0.01] significantly decreased CPP in comparison with morphine group. The results demonstrated that 130-Hz electrical stimulation of the rat AC nucleus significantly blocked the morphine-induced CPP.
Effects of exposure to high-frequency deep brain stimulation (DBS) on morphine-induced conditioned place preference. Exposure to DBS attenuated morphine-induced CPP compared with the morphine group. Data are presented as mean ± SEM. &&& P < 0.001 different from the sham group. @@ P < 0.01 different from the morphine group.
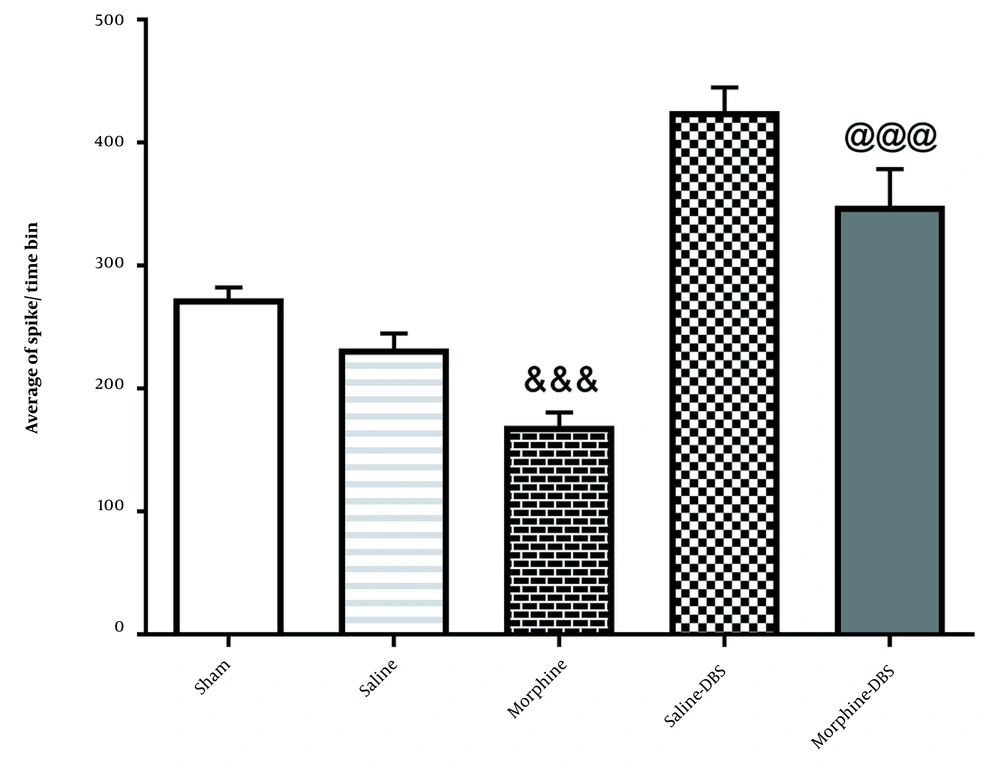
4.2. The Effect of Morphine and DBS on the Average a Spike of Neurons in the Cortical Part of the AC Nucleus in Different Groups
As illustrated in Figure 4, the injection of morphine significantly reduced the average spike in cortical neurons of the AC nucleus [227.7 ± 10.83] compared to the sham group [273.4 ± 8.8] [F4, 25=63.7; P < 0.001]. However, it was increased significantly in the morphine+DBS group [425.7 ± 19.25] compared to the morphine group [227.7 ± 10.83; P ≤ 0.01].
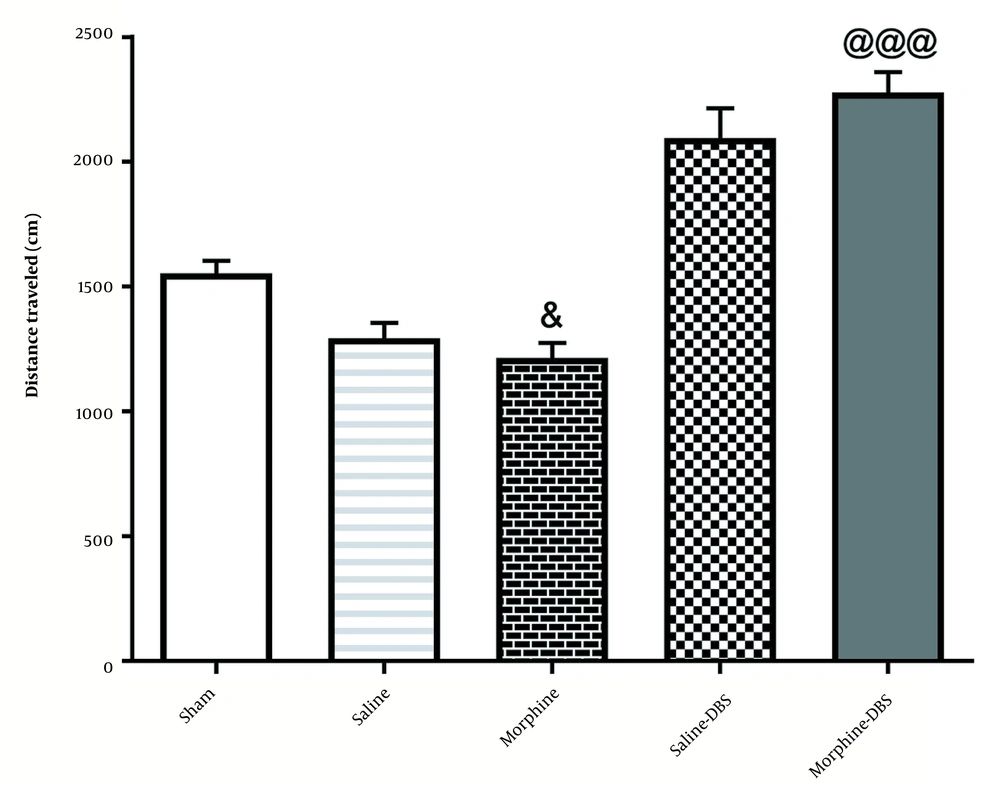
4.3. Locomotor Activity in Different Groups Was Tested 24 h After the last Conditioning Session
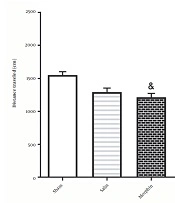
According to Figure 5, administration of morphine without electrical stimulation [935.7 ± 91.60] decreased the total traveled distance compared to the sham group [1553 ± 50.32;] [F4, 35=36.6; P < 0.05]. However, a significant difference was observed in the total traveled distance in the morphine+DBS group [2279 ± 81.06] compared to that in the morphine group [F4, 35=36.6; P < 0.001].
5. Discussion
This study showed that morphine with concentrations of 5 and 10 mg/kg was able to create dependency in the rats, manifested in the occurrence of preferential behavior for the place of receiving it compared to the place of receiving saline. The incidence rate of this dependency in our study was consistent with the rate documented in other studies by Azizi et al., which showed that these doses of morphine in CPP tests may have created dependency (17). In our study, moreover, it was revealed that the administration of DBS in mPFC inhibited CPP caused by morphine with a different effect.
Treatment of the addiction to drugs such as heroin and cocaine is not effective in most cases, and a high percentage (over 80%) of the patients relapse after a few days or years of abstinence. Human studies have shown that this return to substance use can occur either after encountering the substance and the peripheral signs related to it, or after the stress.
The AC nucleus is one of the most important brain regions involved in reward and addiction, which plays a significant role in returning to drug use. Several studies have shown that the AC nucleus is one of the main sites associated with drug use (18-20). One of the inputs of the AC nucleus is the prefrontal cortex. Several approaches have been proposed for treating neurological diseases, among which DBS is one of the most effective approaches. Therefore, the present study aimed to examine the effects of deep stimulation of the prefrontal cortex on the electrical activity of AC neurons as well as on morphine-induced dependency.
According to our study results, DBS significantly reduced the morphine-induced CPP in rats. Furthermore, morphine+DBS had the potential to significantly increase the basal activity of neurons in the cortical part of the AC nucleus. It was found that performing electrical stimulation for three days reduced the preference score in the CPP test compared with that in the morphine group.
The contemporary era of DBS emerged in 1987, and since then, it has been adopted as a suitable strategy for treating a wide range of neurological disorders (21). As for the application of DBS, it induced a functional ‘lesion’ instead of an irreversibly destructive lesion. There are some controversial hypotheses to explain the functional role of DBS, the most important of which are synaptic inhibition, depolarization blockade, synaptic depression, and regulation of pathological network activity. Animals – rats, in particular – play crucial roles in explaining the mechanism by which DBS exerts the ameliorating effect on Parkinson's disease (PD) symptoms. In this regard, electrical stimulation of the subthalamic nucleus can improve the symptoms of PD in humans and non-human primates (22). DBS is considered a sound strategy for treating some neuropsychiatric disorders. However, DBS is a technical innovation that has been used for researching drug abuse. Several reports have indicated that electrolytic lesions to the AC nucleus have remarkable positive effects on conditioning cues of appetitive procedures (23-25). In our study, mPFC DBS with a frequency of 130 Hz and amplitude of 0.2 to 0.5 mA increased neuronal firing of the AC nucleus, which decreased following morphine administration. It was argued that this occurred due to the activation of recurrent inhibitory processes within the mPFC, which are selectively potentiated during DBS. The high-frequency stimulation (HFS) of the nucleus AC in rats blocked the morphine-induced CPP as the measure of morphine reinforcement (26) and inhibited the morphine-induced reinstatement of morphine-seeking in animal models (27). Seemingly, the AC nucleus core allocated powerful value for separate stimuli related to reward or aversion. Furthermore, it is crucial to adapt these values to the changes in circumstances, which leads to the activation of the AC nucleus for induction of addictive relapse behavior (28, 29). Applying high-frequency stimulation of the AC nucleus during the abstinence phase also reduced the restoration of drug-seeking in rats with heroin self-administration (30). Stimulation of nucleus AC in high frequency over the restoration session mitigated the cocaine return of drug-seeking in rats (31). Therefore, Pelloux and Baunez argued that the hypothalamus was not a proper option for the utilization of DBS in drug addiction (32). Liu et al. (cited in Yang et al.) indicated that HFS application limited the morphine-induced preference enhancement (33).
Also, the administration of DBS in the mPFC region increased the reduced motor activity caused by morphine in the open field test. According to the results, DBS may have been employed for treating morphine- induced addiction. Hamilton et al. confirmed that the stimulation of the AC nucleus in a high-frequency current produces more favorable results than the stimulation of it in a low-frequency current regarding the prevention of relapse of the cocaine-induced over addiction withdrawal phase (34). The bilateral stimulation of the AC nucleus by high-frequency current inhibited the return to morphine-seeking behavior in the CPP test. It accelerated the impairment of drug tendency rate in rats with morphine preference (27). Studies showed that the separable effect of electrolytic lesions to the AC nucleus core and shell on conditioning and shell lesion (not core lesion) significantly reduced the function of locomotion and exploratory (35).
Our study faced a few limitations, one of which was the identification of appropriate stimulation patterns for DBS. also it is recommended that future studies should be carried out to investigate the possible effects of DBS on ion-channel currents, especially on potassium ion currents, by using the patch clamp recording method.
5.1. Conclusions
The stimulation of nucleus AC in high frequency inhibited the relapse of morphine place preference after withdrawal, facilitated its impairment, and prevented the return to morphine-seeking behavior in the CPP test. Therefore, it was concluded that this treatment enhanced the locomotor activity and the primary neuron activity in the cortex of the AC nucleus.