1. Background
Malaria, a life-threatening blood disease caused by the Plasmodium parasite, is transmitted by female Anopheles mosquitoes, which carry the infective sporozoite stage of the Plasmodium parasite in their salivary gland (1, 2). Despite intensive attempts to control malaria, it has remained a major public health problem, particularly in tropical countries. It places a major burden on the economy of households and the health sector, as well as on worldwide development (3, 4). About 219 million malaria cases were reported in 2017 worldwide, with an estimated death rate of 435,000 (5). Globally, about 306,000 children under five years died of malaria. Malaria also accounted for almost 3% of disability adjusted life years (DALYs) in 2013 (6, 7).
To combat the scourge of malaria, many approaches, such as prompt treatment, proper diagnosis, and vector control, have been put in place. In the area of vector control measures, environmental manipulation and managements were practiced. To prevent the development of larvae and their emergence to the adult stage, measures such as the introduction of molecular films and oil, insect growth regulators, predatory fish, and bacteria species (Bacillus spp) into water bodies were adopted in the past (8-10). However, their effectiveness was limited to permanent bodies of fresh water. Studies have not affirmed a reduction in the entomological inoculation rate (EIR) and new malaria cases (11, 12).
Anopheles funestus and Anopheles gambiae sensus stricto are the major Plasmodium vector species, which cause malaria disease. Most of these vector species are endophagy, biting at night, and enodphilic for the subsequent hours or days. Residual insecticide sprayed indoors on walls and ceilings (IRS) as well as the insecticide treated net (ITN) was used by the World Health Organization (WHO), in collaboration with the United Nations Children’s Emergency Fund (UNICEF), the United Nations Development Program (UNDP), and the World Bank, as the focus of Roll Back Malaria Global Partnership to coordinate efforts in fighting malaria (13).
Despite the widespread information about the distribution of ITNs/long lasting insecticidal nets (LLINs) for malaria control, many people do not sleep under ITNs/LLINs. Some people find sleeping under LLINs uncomfortable, while others habitually stay up late either to recreate, socialize, or study. For some, the nature of their work does not allow sleeping under the net or requires them to go to bed late. Moreover, there is still poor access to LLINs due to insufficient supply per household and low utilization by those who possess the nets (14-18). In developing novel malaria control methods, insecticidal paints have been introduced and are commercially available. However, it is necessary to assess the efficacy of insecticidal paints and monitor the duration of their effectiveness when used.
2. Objectives
Thus, this study aimed to assess the efficacy of insecticidal paints against Anopheles mosquitoes transmitting the malaria parasite and also the duration of their effect.
3. Methods
3.1. Study Area
The study was carried out in Ikeji, Arakeji (7.508ºN, 4.881ºE), a rural agrarian community in the Oriade local government area of Osun State. A majority of settlers in this community belong to the Yoruba ethnic group. It had a population of about 18,840 people during the National Census of 2006 (19). The two major roads into the town were the only roads laid with asphalt. Other roads were untarred and without drainages. There are pot holes and gullies in the area created by erosion.
3.2. Preparation of Study Rooms
Four rooms (3 x 3.6 square meters each) were painted with an insecticidal paint produced by Inesfly(R) Africa Limited. The paint is composed of 1.5% Chlorpyriphos, 1.5% Daizinon, and 0.063% Pyripyroxyfen, which serve as an insect growth regulator. Two layers of the paint were applied to the walls of the study rooms. Moreover, another four rooms were painted with emulsion paint without insecticide (control). The walls were painted with two layers of the paint. The study was conducted longitudinally for a year.
3.3. Pyrethroid Spray Catch
Pyrethroid Spray Catch (PSC) was performed to determine the mosquito density in the eight experimental rooms between 6-8 am prior to paintings, as described by WHO (20).
3.4. Collection of Anopheles mosquito larvae
Anopheles gambiae s.l. larvae were collected randomly from breeding sites using the dip method (20, 21). They were then transported and sorted into third and fourth instar larvae at the insectary section of the Biological Sciences Laboratory, the Joseph Ayo Babalola University, Ikeji, Arakeji. They were maintained at 28ºC and ~80 humidity with a 12 hr day/night cycle (22) and fed with 12.30 g of a mashed low-fat biscuit and 7.59g yeast capsules (23). The emerged mosquitoes were taken from the bowls with a mechanical aspirator and introduced into plastic cages covered with fine mesh for five days.
The larvae of the Kisumu strain of Anopheles mosquitoes was obtained from the Parasitology and Entomology Laboratory of the Nigerian Institute of Medical Research, Yaba Lagos. The larvae were maintained in the laboratory of Joseph Ayo Babalola University.
3.5. Cone Bioassay for Susceptibility Test
The five-day-old, non-blood fed female adult Anopheles mosquitoes were used for cone bioassay.[24] Polyvinyl chloride cones (PVCs) were fixed on the walls of the experimental rooms, with one PVC cone on each wall side of each of the rooms (Figure 1). Twenty mosquitoes were released into each of the cones using a mechanical aspirator and allowed to stand there for 30 minutes. The number of knocked-down mosquitoes were recorded at time intervals of 10, 15, 20, and 30 minutes (24). After 30 minutes, the mosquitoes were transferred into 150 ml holding cups (10 individuals per cup) covered with a net. A cotton wool pad soaked with 10% sugar solution was placed in each cup so that any surviving mosquito could access sugar (24). The process was also performed for the Kisumu strain. The number of deaths in each cone was recorded 24 hours after the mortality rate was determined from the pool of dead mosquitoes (24, 25). That is,
A total of 11,520 mosquitoes were used for the longitudinal study. The study was conducted monthly for 12 months.
3.6. Statistical Analysis
The data were analyzed with Microsoft Excel 2013. The chi-square test was used to determine the difference in the susceptibility of the mosquito strains. Levels of statistical significance were measured at the 95% confidence interval (CI). The observations were taken to be statistically significant at P value < 0.05.
4. Results
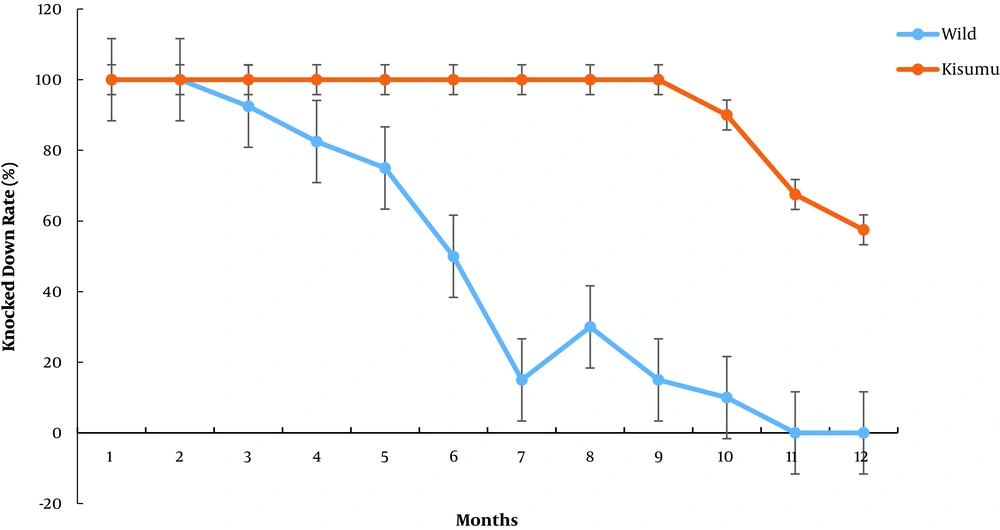
Five mosquitoes were obtained from PSC before painting, while no mosquito was found in the rooms seven days post painting. A knock-down rate of 100% was observed for the wild and Kisumu strains in rooms painted with the insecticidal paint seven days post painting within 10 minutes of exposure. Moreover, a knock-down rate of 100% was observed for the mosquitos within 10 minutes of exposure in the first two months of the paint bioassay. The knock-down rate declined to 92.5% for the wild strain within 10 minutes of exposure in the third month, whereas it remained 100% for the Kisumu strain. However, there is no significant difference between the wild and Kisumu strains in terms of the knock-down rate (X2 = 0.3, df = 1, P = 0.6). In the fourth-month post painting, the knock-down rate declined to 82.5% for the wild strain , although it was not significantly different (X2 = 1.6, df = 1, P = 0.2) from that for the Kisumu strain, which was 100% (Figure 2).
The difference between the knock-down rates for the wild and Kisumu strains became significant in the sixth-month post painting (X2 = 16.7, df = 1, P = 4.5 x 10-5). In the eleventh month post painting, the knock-down rate declined to 0.0% for the exposed wild mosquitos (X2 = 67.5, df = 1, P = 2.1 x 10-16), and also, to 0.0% in the twelfth month of exposure (X2 = 57.5, df = 1, P = 3.4 x 10-14) (Figure 2). Further, the knock-down rate for the Kisumu strain declined from 90% in the tenth month and 67.5% in the eleventh month to 57.5% in the twelfth-month post painting (Figure 2).
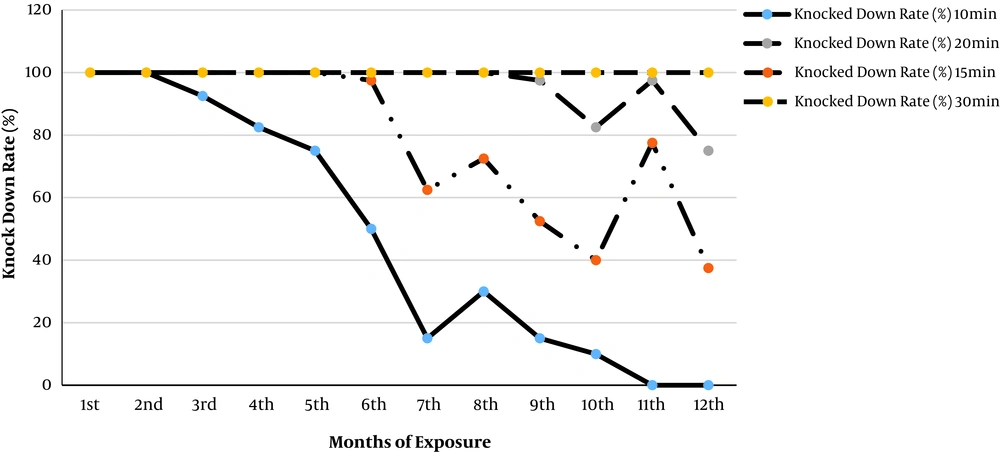
The total knock-down of the wild strain was observed after 15 minutes of exposure in the third to fifth month of exposure. There was also an extension of the total knock-down duration to 20 minutes in the sixth to the eighth month. However, the total knock-down duration was extended to 30 minutes of exposure in the ninth to twelfth month (Figure 3). None of the mosquitoes in the holding cups revived within 24 hours despite the provision of sugar solution in the holding cups.
5. Discussion
The organophosphate-based insecticidal paint was found to be effective, and total knock-down was observed for mosquitoes exposed to it. The total knock-down of mosquitoes (the wild and Kisumu strains) exposed to the insecticidal paint was observed within 10 minutes of exposure until the second month post painting. However, mosquitoes exposed to walls painted with the paint without insecticide were not knocked down. The short knock-down duration indicates the susceptibility of mosquitoes in the study environment to organophosphate insecticide. However, Awolola (26) reported the resistance of mosquitoes in Osun State to other the WHO approved classes of insecticide. The ability of a paint to release insecticide to effectively knock down mosquitos in a short time is a desirable outcome for controlling the malaria vector.
Painting walls with such paints will break the transmission of malaria parasites by the mosquito vector.
Although the insecticidal paint was effective in knocking down mosquitoes, its efficacy began to decrease after the first two months. The knock-down duration to achieve a 100% knock-down rate was observed to extend to 15 minutes from the third to fifth month, 20 minutes from the sixth to eighth month, and 30 minutes from the ninth to twelfth-month post painting. This suggests that the insecticidal paint did not totally lose its potency and only required an extended exposure period. The lethal effect of the insecticidal paint in this study is in consonance with the study conducted by Mosqueira et al. (27).
Retention of potency by the insecticidal paint was further confirmed by a mortality rate of 100% for the exposed mosquitoes obtained throughout the longitudinal study. WHO (1) determines the mortality rate between 98% - 100% as the index of the susceptibility of mosquitoes to insecticide. Therefore, the mortality rate obtained in this study was an indication of the susceptibility of mosquitoes to insecticide implanted in the paint. The high mortality rate found in this study is also in agreement with the research conducted by Mosqueira et al (28) where a high mosquito mortality rate was found after exposure to the insecticidal paint.
The zero mortality rate among mosquitoes exposed to walls painted using a non-insecticidal paint, in contrast to the 100% mortality rate among mosquitoes exposed to walls painted with the insecticidal paint, shows that the variable responsible for the knock-down of mosquitoes was insecticide in the paint, and not chemicals used for the production of the paint. Therefore, the use of insecticidal paints will be an effective intervention for malaria vector control and will provide protection for those unable to sleep under LLINs during mosquito biting hours.