1. Background
Pseudomonas aeruginosa is a gram-negative, oxidase-positive, non-fermenting, and aerobic bacterium found on intestinal tissues of healthy individuals, various fluids, and surfaces, especially moist surfaces and even disinfectants (1). P. aeruginosa is an opportunistic bacterium identified as one of the most critical hospital pathogens in recent years (2, 3). One of the pathogenic mechanisms of this bacterium is the quorum sensing system and high resistance to most antibiotics. Quorum sensing (QS) is the ability to detect and respond to cell population density by gene regulation. It enables bacteria to restrict the expression of specific genes to the high cell densities, at which the resulting phenotypes will be the most beneficial (4, 5). Many virulence genes in P. aeruginosa are controlled and expressed by quorum sensing as biofilm growth and proliferation genes, alkaline proteases, pyocyanins, and pivorinins (4, 5). A biofilm comprises any syntrophic consortiums of microorganisms in which cells stick to each other and a surface (6). The low density and low diffusion power of the matrix polysaccharide existing in the biofilm provides ideal conditions for the aggregation of signals and the induction of pathogenic factors by the coenzyme sensing phenomenon and prepares bacteria to invade the host. The growth of bacteria in biofilms can increase their resistance to the antibiotics. On the other hand, the overuse of antibiotics in the treatment of bacterial infections has led to the development of antibiotic-resistant strains (5, 6). The process of bacterial resistance to chemical antibiotics has limited the physicians to treat some infectious diseases that are often fatal (7). The study of medicinal plants has become particularly critical worldwide to discover new therapies with fewer side effects and higher economic values. Rosemary extract is a compound with several antimicrobial and antioxidant properties
It has been proven that there are numerous antimicrobial compounds, such as phenolic compounds. Today, over 4 billion plants worldwide are used as a source of medicines, and 25% of physicians prescribe normal herbal medicines (8). Plants have an unlimited ability to synthesize phenolic compounds, their derivatives, and a variety of aromatic compounds. These compounds are secondary metabolites of plants and have therapeutic effects against viruses, bacteria, and fungi (8). Therefore, it is necessary to investigate the active constituents of medicinal plants in different geographical areas to discover useful antimicrobial agents. Rosmarinus belongs to the mint family (Labiateae, a herb with green, aromatic, and sharp leaves) and has antinociceptive, antioxidant, antimicrobial, and anti-inflammatory effects on laboratory experiments and antimicrobial compounds (9, 10). It contains phenolic compounds such as carnosol, rosmarinic acid, caffeic acid, flavonoids, including diosmin, luteolin, gencuanine, and monoterpene, such as camphor, cineole, and borneol (10, 11).
2. Objectives
Therefore, the study aimed to evaluate the antibacterial and anti-quorum sensing activity of Rosmarinus methanol extract against Pseudomonas aeruginosa.
3. Methods
3.1. Identification and Collection of Rosmarinus
This experimental study was performed from May 2017 to January 2018 in the Microbiology Laboratory of Kashan Azad University. In spring 2017, leaves and branches of Rosmarinus from rangelands of Niassar city of Kashan were harvested and approved by Isfahan Agricultural and Natural Resources Research Center (12).
3.2. Preparation of Bacterial Strains
In this study, five clinical isolates of P. aeruginosa were isolated from clinical samples referred to Kashan hospitals. Standard bacterium P. aeruginosa (ATCC 1074) was also used. Bacterial samples were cultured in Muller-Hinton broth medium and incubated at 37°C for 24 hours. Subsequently, a few drops of the bacterial suspension were transferred to the Muller Hinton broth medium to achieve a standard McFarland 0.5 turbidity (1.5 ×108 cfu/mL) (13).
3.3. Preparation of Various Dilutions and Evaluation of Antibacterial Activity of the Extract
Concentrations of 500, 250, 125, 62.5, 31.25, 15.6, 7.8, and 3.9 mg/ml of extract were prepared. In this study, the antimicrobial activity of the extract was evaluated by diffusion method in wells and microdilution. After preparation of different concentrations of extract, Measurement of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined by microtiter plate method (14).
3.4. Investigation of the Anti-Quorum Sensing Activity of Ethanol and Methanol Extract of Rosmarinus
We examined the anti-biofilm activity, pyocyanin production, and protease enzyme activity of P. aeruginosa to investigate the antimicrobial sensing activity of methanolic extracts of Rosmarinus.
3.5. Evaluation of Anti-Biofilm Activity of Rosmarinus Methanol Extract
The anti-biofilm activity of Rosmarinus methanolic extract was evaluated by crystal violet staining to investigate its anti-quorum sensing activity.
3.6. Inhibition of Pyocyanin Experiment
The 24-hour culture of the bacteria was prepared in the Muller Hinton broth medium and its opacity was set to half the standard McFarland opacity by spectrophotometer. 250 µl of the herbal extract was prepared at a concentration lower than the bacterial growth inhibitory, and 100 µl of microbial suspension was added. The test tube containing microbial suspension without extract was considered as control. The tubes were centrifuged at 8,000 rpm, and the supernatant was transferred to another sterile tube and added to 3 ml of chloroform. The solution was then transferred to the cuvet from the bottom of the tubes (control and extract-treated samples) and read by a spectrophotometer at 690 nm. This experiment was repeated three times, and the mean percentage reduction of pyocyanin production was calculated according to the following formula (15).
Percentage reduction of pyocyanin = (Optical Absorption of extract - Treated Sample - Optical Absorption of control sample)/(optical absorption of control sample) ×100
3.7. Protease Activity Test
Protease activity of P. aeruginosa was evaluated in accordance with the Lori method using azocasein (15).
3.8. Statistical Analysis
The comparison of the means of this study was analyzed by two-way ANOVA and Bonferroni paired t-test using SPSS 17 software. The significance level of the test (P < 0.05) was used to interpret the data.
4. Results
In this study, the strains of P. aeruginosa were identified by hot staining and conventional biochemical tests. All isolates were Gram-negative bacilli, catalase-positive, and oxidase-positive cultured in oxidation-fermentation (OF) medium. These isolates produced pyocyanin were mobile and grew at 42°C. The susceptibility of clinical isolates and standard strains of P. aeruginosa to methanolic extracts of Rosmarinus was investigated by the well diffusion method (Table 1).
| Sample Concentrations (mg/ml) | 500 | 250 | 125 | 62.5 | Positive Control (Imipenem) |
|---|---|---|---|---|---|
| P.aeruginosa (ATCC:1074) | 19.75 ± 0.95 | 18.50 ± 1.00 | 14.25 ± 1.50 | 11.5 ± 1.00 | 28.00 ± 1.81 |
| P.aeruginosa isolate 1 | 18.75 ± 0.50 | 16.50 ± 0.50 | 13.00 ± 1.00 | 9.80 ± 0.76 | 28.00 ± 2.12 |
| P.aeruginosa isolate 2 | 17.50 ± 0.50 | 13.50 ± 0.50 | 10.50 ± 0.50 | 8.50 ± 0.50 | 27.00 ± 2.12 |
| P.aeruginosa isolate 3 | 19.75 ± 0.50 | 17.25 ± 0.50 | 14.25 ± 1.50 | 10.80 ± 0.76 | 28.00 ± 2.12 |
| P.aeruginosa isolate 4 | 16.50 ± 0.50 | 14.00 ± 0.50 | 10.80 ± 0.28 | 7.60 ± 0.57 | 26.00 ± 1.94 |
| P.aeruginosa isolate 5 | 22.50 ± 1.00 | 21.25 ± 0.95 | 15.00 ± 0.00 | 11.80 ± 0.28 | 30.00 ± 1.81 |
Table 1 shows the inhibition zone mean of methanolic extract of Rosmarinus plant on different isolates of P. aeruginosa (in mm). As the two-way ANOVA test shows, the size of the inhibition zone diameter is directly proportional to different concentrations (P < 0.001). The minimum inhibitory concentration of methanolic extract of Rosmarinus was 125 mg / ml, and minimum bactericidal concentration was 250 mg/ml.
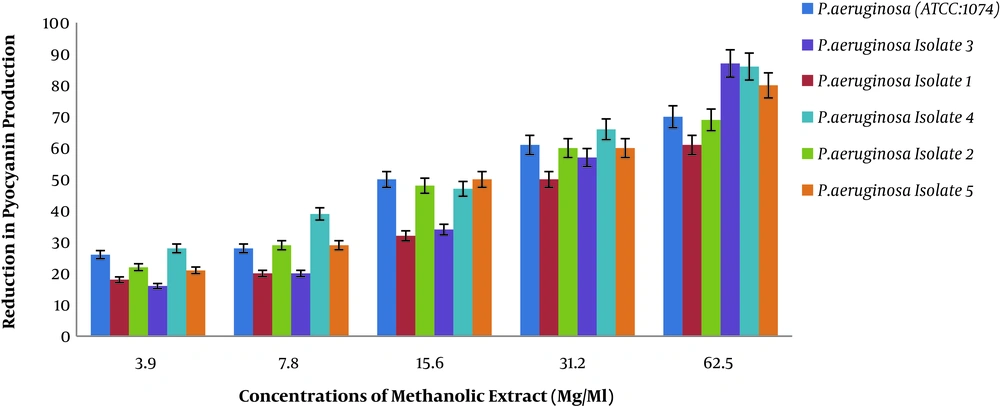
4.1. Results of Pyocyanin Pigment Reduction in P. aeruginosa at Different Concentrations of Methanolic Extract of Rosmarinus
As presented in Figure 1, we evaluated the efficacy of methanolic extract of Rosmarinus on the reduction of pyocyanin pigment production of different P. aeruginosa isolates.
Figure 1 shows a decrease in pyocyanin pigment production in the presence of different concentrations of Rosmarinus methanol on different P. aeruginosa isolates. Two-way analysis of variance showed that the percentage of reduced pigment production of piocyanin was directly correlated with different concentrations of the extract (P < 0.001). In other words, with increasing methanol concentration of Rosmarinus, pyocyanin pigment production can decrease in standard P. aeruginosa and clinical isolates, which means that methanolic extract of Rosmarinus can strongly significantreduce pyocyanin pigment production in Pseudomonas aeruginosa.
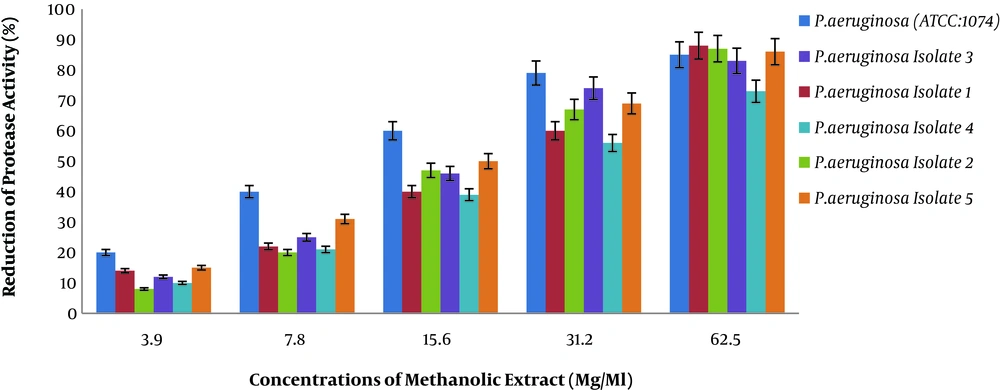
4.2. Results of Percentage Reduction of P.aeruginosa Protease Activity at Different Concentrations of Methanolic Extract of Rosmarinus
We assessed the efficiency of methanolic extract of Rosmarinus on reducing the protease production of different isolates of P. aeruginosa used by Lorry method, as presented in Figure 2.
Figure 2 shows a decrease in protease production in the presence of different concentrations of methanolic extract of Rosmarinus plant on different isolates of P. aeruginosa. Two-way analysis of variance showed that the percentage of reduced protease production was directly correlated with different concentrations of extract (P < 0.001). In other words, as the concentration of methanolic extract of Rosmarinus increases, the production of protease in standard P. aeruginosa and clinical isolates has decreased, which means that methanolic extract of Rosmarinus has a significant effect in reducing the production of protease in P. aeruginosa.
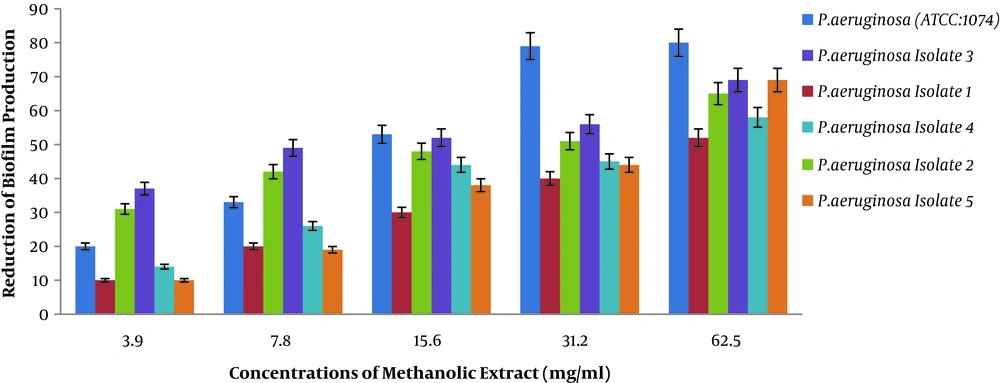
4.3. Results of Anti-Biofilm Activity of Methanolic Extract of Rosmarinus
In the present study, we examined the anti-biofilm activity of methanolic extract of Rosmarinus via crystal violet staining on P. aeruginosa (Figure 3). Figure 3 shows the reduction of biofilm production in the presence of different concentrations of methanolic extract of Rosmarinus plant on different P. aeruginosa isolates. Two-way analysis of variance showed that the percentage of reduction in biofilm production was directly correlated with different concentrations of extract (P < 0.001). In other words, with increasing concentration of methanolic extract of Rosmarinus, biofilm production in P. aeruginosa decreased, and clinical isolates has decreased, which means that methanolic extract of Rosmarinus has a significant effect on reducing biofilm production in P. aeruginosa.
5. Discussion
The current study investigated the antibacterial and antibacterial activity of sensitizing Rosmarinus methanol extract against standard and clinical P. aeruginosa strains. The results showed the inhibitory effect of methanolic extract of Rosmarinus on the growth of clinical isolates and standard P. aeruginosa (ATCC 1074). Also, the results of the mean comparison of different concentrations of the extract showed that with increasing concentration of extract, antibacterial activity increased, and the size of non-growth zone diameter was directly proportional to the concentration of extract (P < 0.05). The methanolic extract of Rosmarinus also prevented the binding and weakening of the isolates of P. aeruginosa isolates for biofilm production on the plate of ELISA wells, which means that the Rosmarinus methanol extract has anti-biofilm properties. The methanolic extract of Rosmarinus at concentrations above 62.5 mg/ml not only reduced pollution but also prevented biofilm formation, protease production, and pyocyanin P. aeruginosa. Studies have shown that some plants produce compounds that are capable of affecting bacterial molecular signals and inhibiting behaviors under the control of the quorum-sensing phenomenon. In this regard, preliminary investigations have confirmed the inhibitory effect of methanolic extract of Rosmarinus on the growth of bacterial and biofilm bacteria.
In 2016, Ahmady-asbchin investigated the inhibitory effects of Rosmarinus essential oil on the bacteria. The results showed that Rosmarinus essential oil had a bacteriostatic effect on E. coli in dilution 1: 8 and a bacteriocidal effect in dilution 1:4 (15). In a 2020 study, Nakagawa et al., investigated the antimicrobial effects of rosemary extract on Staphylococcus aureus and reported a MIC of 5 mg / ml of the extract (16). In 2013, Golshani et al. reported a minimum inhibitory concentration for Staphylococcus aureus of about 6.25 mg/ml (17). Sandasi et al. (2011) examined the potential of inhibiting the growth of P. aeruginosa biofilm by in-vitro treatment of eight plant extracts. Their results indicated the extract of Rosmarinus as having the highest antimicrobial activity with a MIC of 1.5 mg/ml. Also, most extracts reduced microbial colonization by 50% (18). In 2017, Oliveira et al. reported a minimum inhibitory concentration of Rosmarinus extract on P. aeruginosa (ATCC 15442) at 6.25 mg/ml. The methanolic extract of Rosmarinus at concentrations lower than the inhibitory can reduce the production of P. aeruginosa biofilms by 98.23% (19). Ceylan et al. (2014) studied the minimum inhibitory, and lethal concentrations of Rosmarinus extract for P. aeruginosa MU 187 as 20 and 80 μg / ml, respectively. At the concentration of 80 µg/ml 74.71%, the inhibitory effect of biofilm formation was observed (20). Sternia et al. (2020) showed a combination of rosemary extract and buffered vinegar inhibiting the growth of various species of Pseudomonas, and the MIC of the extract was reported to be 3.13 mg/ml. (21). Jawad et al. (2018) and Tural et al. (2019) examined the antimicrobial effect of rosemary extract on P. aeruginosa. Accordingly, the mean diameter of the growth inhibition zone of rosemary extract against Pseudomonas was equal to 10 and 20 mm, respectively, at a concentration of 128 mg/ ml (22, 23).
Araby et al. obtained the minimum inhibitory concentration of Rosmarinus essential oil for six P. aeruginosa isolates from 5 to 25 μg/ml (24). The results showed that there was a significant decrease in biofilm production, which was consistent with the present study. Wafa et al. (2020) investigated the effects of rosemary extract on the formation of P. aeruginosa biofilm. They showed that 17 (85%) of the total P. aeruginosa isolates could produce biofilm. Rosemary extract was the most useful extract in biofilm inhibition, inhabiting 83% of P. aeruginosa biofilm (25). According to the chemical analysis, the antimicrobial compounds of the Rosmarinus mainly included thymol, flavonoids, triterpenoids, and other compounds with the nature of a phenolic or free hydroxyl group, all known as the most active antimicrobial and antibiofilm compounds. According to the studies, as secondary plant metabolites react with a wide range of cellular components, these compounds tend to influence a large number of cellular targets. Most extracts are believed to exert their antimicrobial activities through interaction with bacterial cell membrane processes, including electron transfer, ionic gradient, protein translocation, phosphorylation, and other enzyme-dependent reactions (26). Also, some differences in the results of these studies are related to differences in plant growth, amount of useful substances, and type of microorganism, extract, harvest season, and different methods of investigation.
The next phase of the present study examined the antiprotease and pyocyanin activity of methanolic Rosmarinus extract on P. aeruginosa. Here, the methanolic extract of Rosmarinus at a concentration of 62.25 mg/ml reduced 85% and 70% of protease activity and production of pyocyanin pigment in P. aeruginosa, respectively. In the Korkorian study, the total protease activity of P. aeruginosa isolates was reduced by 27% Rumex alveolatus sub-lethal dilution (27). Sankar Ganesh et al. (2015) and Araby et al. (2016) demonstrated that Rosmarinus essential oil caused a significant decrease in the production of pyocyanin in P. aeruginosa isolate (24, 28). The results of the present study also indicated that methanolic extract of Rosmarinus could reduce the activity of protease enzyme and the production of pyocyanin pigment in bacterium and reduce its pathogenicity.
5.1. Conclusion
Mentioning the above bacteria, the present research tended to confirm the efficacy of the methanol extract. The results could verify the ability of Rosmarinus methanol extract to reduce microbial growth, biofilm, elastase, protease, and pyocyanin of P. aeruginosa. Given the high potential of P. aeruginosa in biofilm formation and the microbial and biofilm growth inhibitory activity of Rosmarinus extracts, it can be concluded that Rosmarinus extract can be used in different compounds for the elimination of infection with pathogenic bacteria such as P. aeruginosa. Also, it can be a substitute for chemical drugs to treat infections, although more thorough investigation on all the effects of this plant extract. Currently, one of the major problems in the treatment of infections and the use of antibiotics is the development of antibiotic resistance, which requires special attention for treatment. Since the antibacterial effects of rosemary extract have been verified in various studies on numerous species of bacteria, it can be employed in the treatment of infections caused by resistant bacteria. To sum up, the effects of plant extracts on inhibition of biofilm formation can be attributed to the those of constituents on bacterial growth and ultimately on the reduction of biofilm formation. Therefore, further studies are needed to evaluate the variety and composition of essential oils and extracts of medicinal plants and to compare different herbs in terms of their constituents in indigenous regions and identify the superior breeds.