1. Background
Foot-and-mouth disease (FMD) is an infectious and sometimes fatal viral disease (1). This disease influences cloven-hoofed animals, such as domestic and wild bovines (2). FMD has severe implications for animal farming. Since FMD is highly infectious, it can be spread by polluted animals by aerosols, contact with contaminated farming equipment, vehicles, clothing, or feed, and through domestic and wild predators (3).
The virus responsible for FMD is a picornavirus, a member of the genus Aphthovirus. Seven serotypes (4) of this virus are observed including A, C, O, Asia 1, SAT1, SAT2 and SAT3 (5).
FMD results in serious economic losses when outbreaks of the disease occur. Similar to other picornaviruses, FMDV has a single stranded positive RNA molecule (6) encapsidated in an icosahedral capsid made of 60 copies each containing four proteins: VP1, VP2, VP3, and VP4 (7). The virus exhibits a great antigenic variability extensively characterized in tissue culture and in the field (8-11). Of the four structural proteins, only VP1 elicits neutralizing antibodies in animals (12-15). A main antigenic site on FMDV is identified around amino acid residues 140 to 160 of VP1, the extreme carboxy terminus (residues 200 to 213) of that protein likely contribute to the antigenicity (16-19).
Humans are very rarely infected with FMD through contact with polluted animals. The virus that causes FMD cannot spread to humans via consumption of infected meat because it is sensitive to stomach acid. The last confirmed human case happened in 1966 (20-22) in the United Kingdom. Moreover, only a few other cases were recorded in countries of the continental Europe, Africa, and South America. FMD in humans has symptoms including malaise, fever, vomiting, red ulcerative lesions of the oral tissue and sometimes vesicular lesions of the skin. One case reported that FMD killed two children supposedly due to infected milk in England in 1884 (23). Since FMD is on top of the world organization for animal health (OIE) list, early and rapid virus detection has a great importance. Complement fixation test (CFT) was widely used to detect the FMD virus, but it was alternated by ELISA due to its sensitivity and assessment of a lot of samples. At present, reverse transcription polymerase chain reaction (RT-PCR) along with ELISA and cell culture are used for early detection and determination of the type of FMD virus in the world reference laboratory (WRL) (24). The current study aimed to introduce ion exchange chromatography as a convenient method to purify specific antibody against 146S antigen of FMDV serotype A.
2. Objectives
The current study aimed to introduce ion exchange chromatography as a convenient method to purify specific antibody against 146S antigen.
3. Methods
3.1. Virus Source
Foot-and-mouth disease virus serotype A was provided by research and production of foot-and-mouth disease vaccine center of Razi institute, Tehran, Iran. The virus was inactivated by ethylene amine double method and purified by sucrose gradient procedure.
3.2. Immunizing a White New Zealand Rabbit to Produce Specific Antibody
Laboratory animals were provided by production and research center of Razi institute. For immunization, a two-month male rabbit (2 Kg weight) was selected; 120 μL of purified antigen 146S along with 880 μL Tris buffer 0.05 M and 1 mL of the CFA adjuvant in a total volume of 2 mL was prepared as an injection suspension. One injection was performed subcutaneously on the back of the rabbit. After 28 days, the second injection was done, as mentioned above, but this time incomplete Freund’s adjuvant (IFA) was used as an adjuvant. Twelve days after the second injection (40th day), bleeding was performed from heart of the rabbit. To obtain the serum, the samples maintained in the incubator (37°C) for 30 minutes and then located in refrigerator (4°C) for one hour. Finally, the samples were centrifuged at 3000 g for 10 minutes.
3.3. Agglutination Assay
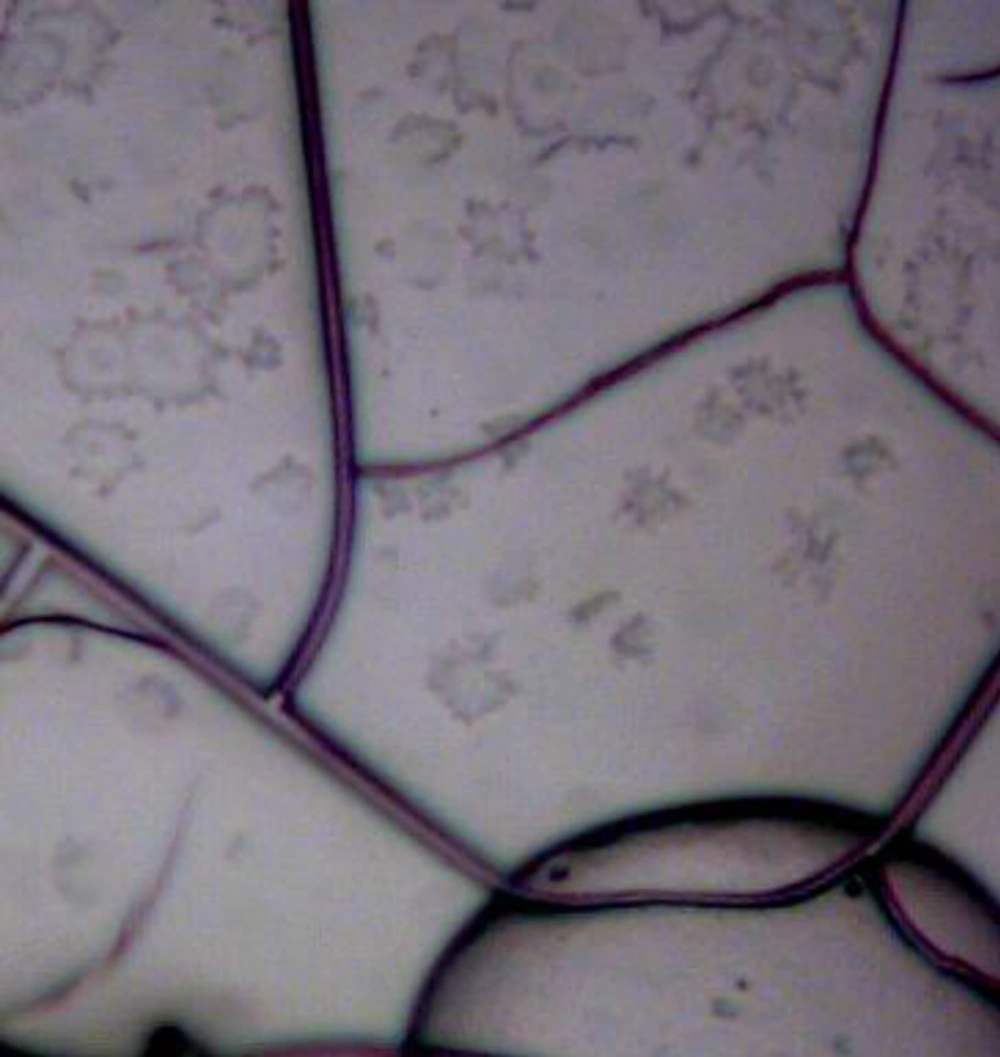
Agglutination reactions produce visible aggregates of antibody-antigen complexes when antibodies or antigens reacting against each other. In this method, one drop of pure antigen was put onto the slide and then one drop of the antibody was added. Due to enhancing of the reaction, slide was shaken. The quality of the result is determined by the time of incubation with the antibody source, amount of the antigen and conditions of the test environment. Optical microscope was used for better observation.
3.4. Dot Blot
Presence of the antigen 146S was confirmed by dot blot; 2 μL of the purified antigen 146S and 2 μL of phosphate-buffered saline (PBS), as a negative control, were spotted on a piece of nitrocellulose membrane with a distance of 1 cm from each other. The membrane was placed in a plastic container and incubated at 37°C for 15 minutes and blocked with PBS containing 1% bovine serum albumin (BSA) (pH 7.4) at room temperature for 45 minutes. After washing with PBS containing Tween 20 (5%); the membrane was incubated with sera (specific antibody solution against 146S antigen) at 1:10 dilution at 37°C for 45 minutes. After washing with PBS-T, the membrane was incubated with the enzyme-labeled secondary antibody (horseradish peroxidase-labeled anti-rabbit immunoglobulin; IgG, 1: 1000) at 37°C for 45 minutes. Then, the membrane was washed again with PBS-T, and the enzyme reaction was developed with 3, 3'-diaminobenzidine (DAB) solution and peroxidase.
3.5. ELISA
The immunized rabbit serum was evaluated for anti-146S-specific antibodies by ELISA. Briefly, flat-bottom 96-well microplates were coated with 150 μL of 146S and incubated at 37°C for one hour. After washing with PBS containing Tween-20 (5%), the plates were blocked with 1% BSA. Then, 150 μL of the immunized rabbit serum was added to each well. The plates were washed and incubated with 150 μL of anti-rabbit IgG-enzyme conjugate (dilution 1: 1000). After washing, enzyme-specific substrate, 2, 2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), was added to each well. The reaction was stopped by 2N sulfuric acid and the absorbance was measured at 490 nm.
3.6. Purification of the Specific Antibody by Ion Exchange Chromatography
In the current study, 25, 2-diethylaminoethyl (DEAE) Cephadex (containing positive charge) was used as a solid phase. The sample was poured into the column. Immunoglobulins have positive charge in pH 9; therefore at the beginning they do not bind to the bead and elute from the column. Then, the column was washed by Tris in different pH levels (8.5, 8, 7.5, 7 and 6). The absorbance of each fraction was measured by spectrophotometer at 280 nm and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was used to observe the protein band.
4. Results
4.1. Agglutination
The interaction of 146S antigen with immunized rabbit led to agglutination reaction. Assay with heterologous antigen showed negative result that confirmed production of specific antibody in the rabbit (Figure 1).
4.2. Dot Blot Assay for the 146S Antigen and the Antibody Elicited from Immunized Rabbit
In the dot blot, presence of brown spots is resulted from binding of specific antibody to 146S antigen. The severity of color depends on the amount of antibody and the purification procedure.
4.3. Specific Antibody Against Serotypes O, A and Asia1 by ELISA
It should be noted that all samples were tested twice and the average of the results was mentioned here for the accuracy of conclusion. The result of ELISA showed that each antigen of different serotypes reacted better with homolog antibody (Table 1). However, to achieve the best dilution of antigen and antibody further testing is required.
| Rabbit | Ab A | Ab Asia1 | Ab O | Blank PBS | |
|---|---|---|---|---|---|
| A | Ag A | 1.288 | 1.014 | 0.695 | 0.286 |
| B | Ag Asia1 | 0.878 | 1.259 | 0.778 | 0.322 |
| C | Ag O | 0.602 | 0.604 | 0.888 | 0.338 |
Confirmation of the Antibody Against Different Serotypes in the Immunized Rabbit and Guinea Pig Serum by ELISA
4.4. Purification of the Rabbit IgG Against 146S Antigen of FDM Virus Serotype A
Since IgG contained positive charge and DEAE Cephadex gel in ion exchange chromatography also had positive charge, IgG was eluted at the first step by buffer (pH9) and the other proteins were eluted by buffer at different pH levels (Figure 3). The amount of absorbance of IgG was 0.197 that showed IgG 0.725 = mg/mL.
5. Discussion
Foot-and-mouth disease is an acute viral disease which is highly contagious and afflicts almost all even hoofed animals. Fever, general malaise, epithelial blisters, weight loss, abortion, limping and death of young animals are considered as main symptoms of the disease (25, 26). FMD is rarely fatal but the diagnosis is significantly important as it spreads fast and widely and brings in great economic damage. FMD is one of the endemic diseases in Iran and costs annual high expenses to defeat (27-29). The ELISA is one of the most valid diagnosis tests which are now available in the reference centers such as WRL. The production of the above kit is done by the laboratories of FMD global reference and is very expensive. This kit is produced by the WRL of FMD which is very expensive. Specific antibodies related to FMD serotypes are coated in this kit; 146S particle of FMD virus is a complete viral particle favorable to neutralize antibodies produced against it. In this complete viral particle, there are four antigenic surfaces; including VP1, VP2, VP3 and VP4. In the current study, the whole 146S particle was used. One of the first steps in the production of diagnostic kits by ELISA technique is purifying antigen 146S of FMDV. In the present study, 20% - 50% sucrose gradients were used for the purification. This antigen was disintegrated into RNA, structural protein (VP4) and also VP1, VP2, VP3 proteins in the PH of less than 6.5. The current study aimed to purify specific antibodies against serotype A of this virus. In the current study, the 146S antigen was used, which includes viral RNA molecules and 60 copies of each VP1-4. The VP4 protein is internal and VP1-3 proteins are located on the surface of the capsid. The VP1-3 proteins as antigenic markers are located on the surface of the virus, but only the VP1 protein can stimulate neutralizing antibodies. In order to purify 146S antigen of FMD virus-serotype A-sucrose gradient method was used. The laboratory animals selected for immunization and antibody production were New Zealand white rabbits weighing 2,000 grams.
In 1980, Cartwright et al. studied the association of serology and immunology between 146S particle and 12S of FMD virus. Tests conducted to confirm the presence of specific antibodies in the sera of immunized rabbits were performed by rapid agglutination on slides, dot blotting and ELISA (30).
In line with the current study, Butcher and McCullough studied the production of monoclonal antibodies against 146S and 12S particles of serotype O of FMD virus. They used sucrose gradient to purify these particles (31).
Gurhan et al. used western blotting and indirect ELISA to confirm the presence of specific antibodies against FMD virus antigen-serotype O (32).
Aggarwal et al. studied the association and specificity of antibodies against the whole particle of FMD virus (146S) -serotype O- with antigenic sites of viral capsid. In the study, ELISA was used to detect specific polyclonal antibodies (33).
The results showed that 12S particles are on the complete viral particle (146S) of FMD virus, there are antigenic sites with similar structure that can stimulate antibody production (33).
In short, purification of 146S antigen of FMD virus was performed by sucrose gradient. The injection methods and production of specific antibodies in the current study were based on the valid reference, WRL. To confirm the presence of antibodies against 146S antigen of FMD virus serotype A, dot blotting and ELISA were applied. To achieve the best result of antigen-antibody dilution additional tests should be performed.