1. Background
Lactoferrin (previously known as lactotransferrin) is a glycoprotein, a member of the transferrin domestic (1). The existence of iron-banding protein in bovine milk was reported in 1939 (2). The bactericidal effect of lactoferrin was well recorded in 1961, as well as its iron-binding capacity. Lactoferrin is a globular glycoprotein with a molecular weight of 80 kDa, widely present in various discharge fluids such as milk, saliva, tear, and nasal secretions. Expression of lactoferrin starts from two or more cells in embryonic stage; it is present in implantation blastocyst stage and then in epithelial cells; it is also present in the immune system, as well as in reproductive and digestive systems. Neutrophils are significant sources of lactoferrin and most parts of the body are sources of neutrophils (3).
There is controversy on the protein base of lactoferrin. Research has shown that its isoelectric point is 8.7. There are two types of lactoferrin: iron-rich holo-lactoferrin and iron-free apolactoferrin. Besides iron, lactoferrin is able to bind large amounts of other compounds and substances such as lipopolysaccharide, heparin, glycosaminoglycans, DNA, or other metal ions such as Al3+, Ga3+, Mn3+, Co3+, Cu2+, Zn2+, etc. The secondary structure of this protein is different; apolactoferrin is specified due to the “open” conformation of the N-lobe and the “closed” conformation of the C-lobe and both lobes are closed in holo-lactoferrin (4).
Lactoferrin has several physiological effects on the body, such as regulation of iron absorption in the intestine, immune response, serving as an antioxidant, anti-carcinogenic and anti-inflammatory agent, and care and protection against bacterial infections (4). Lactoferrin plays a key role in maintaining the cellular iron level in the body. Several studies show that breastfed babies will not encounter iron deficiency (5). Lactoferrin shows antibacterial activity in vitro, because of its ability to bind iron, and thus the iron will become inaccessible to the bacteria (6). Lactoferrin shows antimicrobial activity against the following bacteria: Proteus sp. Yersinia pestis, Streptococcus pyogenes, S. canis, S. agalactiae, Klebsiella pneumoniae, S. zooepidemicus and Candida albicans (7). In vitro antimicrobial activity of lactoferrin has been shown in different studies (8).
There is evidence to suggest that the antimicrobial activity of lactoferrin is much more complex than its iron-binding activity. The presence of iron in the environment is essential for bacterial growth. Lactoferrin binds to iron to make it out-of-reach for the bacteria. Lactoferrin binds to the lipid component of lipopolysaccharide (LPS) and disrupts binding of other components to LPS in the bacterial cell (9, 10). Therefore, it interrupts the pathogenesis of some important enteropathogens by interfering with surface-expressed pathogenesis factors. For example, bovine lactoferrin inhibits Yersinia spp. login in epithelial cells (11). Lactoferrin also causes apoptosis in cells (12). Other enteropathogens have similar complex interactions with lactoferrin. Lactoferrin has antibacterial activity and its iron-binding mechanism can destroy the bacteria (13). Apolactoferrin (iron-free lactoferrin) increases the permeability of the bacterial membrane by directly damaging the outer membrane of Gram-negative bacteria (14-16).
Lactoferrin is the first component of immune homeostasis and plays an important role in the control of cellular oxidative and inflammatory responses. There is considerable evidence that oxidative stress causes many neurological diseases including Parkinson, Alzheimer, amyotrophic, strokes, seizures as well as rheumatoid arthritis, fatigue and cancer. The presence of lactoferrin as a mediator in the control of oxidative stress and its role in immune homeostasis reduce neurological diseases and increase longevity (17).
Due to the physiological functions of lactoferrin in the host immune system and in increasing the production of industrial antibacterial and chemical drugs, we can isolate milk lactoferrin mainly as a natural antibacterial agent and benefit from the lactoferrin gene in more developed organisms such as plants expressions.
2. Objectives
The purpose of this study was to evaluate the effect of lactoferrin on two different species of Gram-negative and Gram-positive bacteria.
3. Materials and Methods
3.1. Proteins
Lactoferrin was purchased from Sigma Corporation (Germany). The purity of the protein was checked by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and was more than 90%. Contaminating endotoxin (lipopolysaccharide) was removed from all the samples using Detoxi-Gel (Pierce Chemical, Rockford, IL USA) and the endotoxin content was then less than 3 EU/mL (250 ng LPS/mg protein or peptide), as measured by the limulus amoebocyte lysate (LAL) assay (Sigma Chemicals, St Louis, MO, USA).
3.2. Bacteria
In our study, all the species of Bacillus cereus, Staphylococcus epidermidis, Campylobacter jejuni, and Salmonella were obtained from different clinical specimens such as wounds, blood, secretions, urine, stool, and sputum from Namazi Hospital of Shiraz. All the bacteria were confirmed by biochemical tests.
3.3. Antibacterial Activity
The bacterial colony counting assays were conducted according to the Clinical and Laboratory Standards Institute (CLSI) and American Society for Testing and Materials (ASTM) G22-76. The bacterial species were suspended in Luria broth (LB) medium and the densities were matched to 0.5 McFarland standard at 640 nm (108 CFU/ mL) and then diluted to 105 CFU/mL with LB. A diameter of 30 mm of lactoferrin was placed in a 10-mL liquid culture containing 10 μL of each bacterial suspension. Thereafter, the samples were incubated at 37°C for 48 hours (shaking incubator, Shin Saeng, FineTech, Korea). From the incubated samples, a 100-μL solution was taken and diluted with the appropriate dilution factor and the final diluted microbial solution; then, it was distributed on nutrient agar plates (Farazbin Kimia Co., Tehran, Iran). The plates cultured without lactoferrin under the same condition were used as controls. All the plates were incubated at 37°C for 24 hours and the number of former colonies was counted. The antibacterial efficacy of lactoferrin was calculated by the following equation (12).

3.4. Statistical Analysis
For all the samples, the experiments were repeated three times. Data were imported to Microsoft Excel and then analyzed using SPSS 21. All the data were studied with mean representational for bacterial strains and completely random design statistics for fungi samples. P < 0.05 was regarded statistically significant.
4. Results
4.1. Antibacterial Activity of Lactoferrin
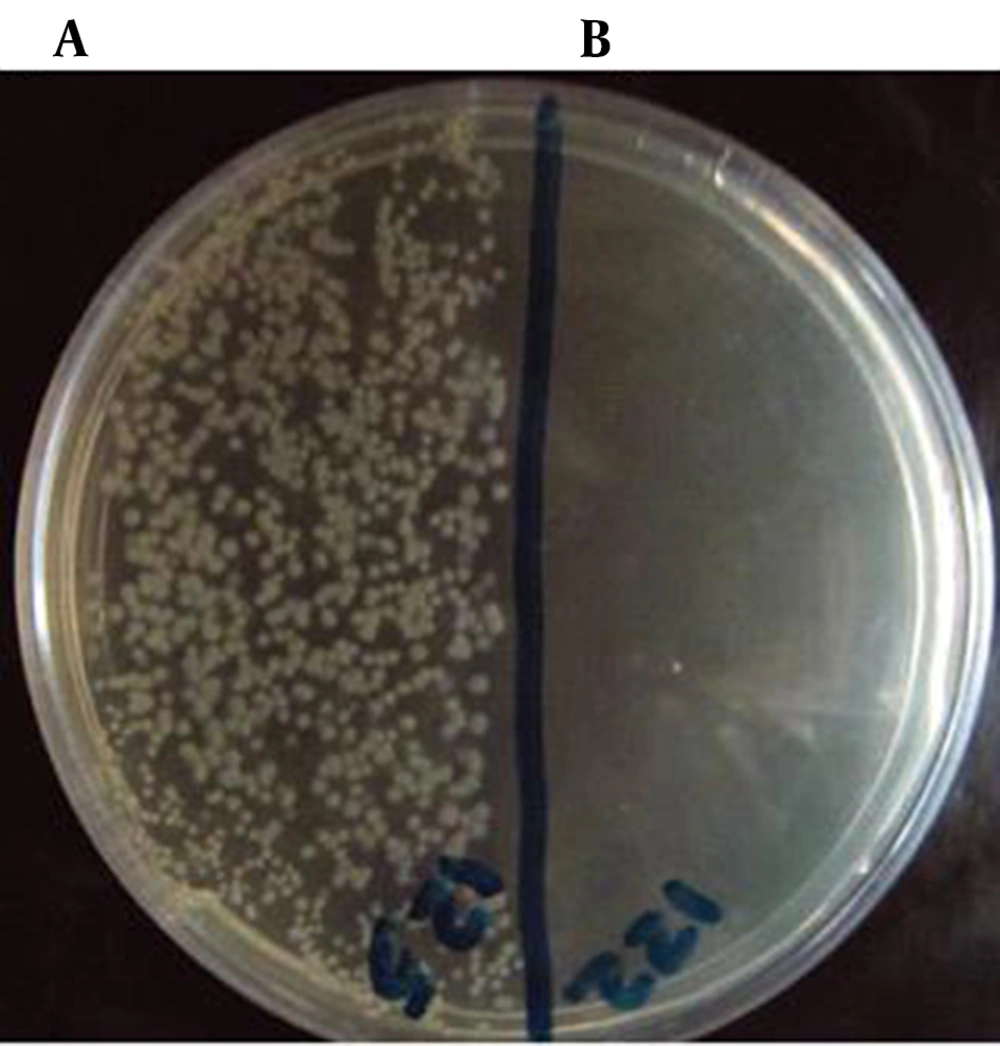
The results showed strong antibacterial effect of lactoferrin on both Gram-positive (S. epidermidis, B. cereus) and Gram-negative (C. jejuni, Salmonella) bacteria; however, it was more effective on Gram-positive rather than gram-negative bacteria. The results are summarized in Table 1. Based on the results, the highest antibacterial activity of lactoferrin was against S.epidermidis, followed by B. cereus, Salmonella and C. jejuni (Figure 1).
| Test | Staphylococcus epidermidis | Bacillus cereus | Salmonella | Campylobacter jejuni |
|---|---|---|---|---|
| Without lactoferrin | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| With lactoferrin | 76 ± 2.5 | 97 ± 2.8 | 35 ± 2.6 | 14 ± 2.7 |
a Statistically significantly different by Duncan’s domain test at P < 0.05.
5. Discussion
In this study, lactoferrin had a noticeable antimicrobial effect on Gram-positive and Gram-negative bacteria. Various studies have examined the antibacterial activity properties of lactoferrin. Lactoferrin is part of the intrinsic safety of the human body. Lactoferrin may indirectly participate in the innate immunity of the body (18). Lactoferrin has a strategic location in the mucosal immune system and body. Lactoferrin secretion in the mucosal tissue makes it the first system to adhere to and deal with microbial agents. Lactoferrin inhibits the growth of Gram-positive and Gram-negative bacteria, viruses and fungi (19). It can bind to free iron, which is essential for the bacterial growth; this binding is responsible for the bacteriostatic effect of lactoferrin (20). Iron deficiency can prevent the growth of bacteria such as Escherichia coli (21). Lactoferrin is able to prevent biofilm formation (by P. aeruginosa) in vitro. Absence of iron in the environment forces bacteria to move; as a result, they cannot adhere to surfaces (22).
Lactoferrin is a natural protein present in milk. It has a great affinity for binding to Iron. Lactoferrin is an essential protein which can inhibit the growth of pathogenic bacteria in stomach and control cell or tissue damages. Lactoferrin is an iron-regulatory system, supporting the functions of the immune system. Iron is a keys ingredient for growth and repair of microorganisms. In the regulation of iron absorption by the gastrointestinal tract, lactoferrin helps to maintain the balance of beneficial and harmful bacteria. Lactoferrin binds to free iron in the body and helps with homeostasis. Lactoferrin provides bound iron to beneficial bacteria and healthy cells through transferrin and helps maintain the surface iron by a complex biological process including transferrin and ferritin. In the absence of lactoferrin, iron will be accessible to pathogens (23).
Lactoferrin might contribute to defense against the attack of various intracellular bacteria by binding to both target cell membrane glycosaminoglycans and the attacking bacterial, which prevents pathogens adhesion to target cells. This feature was for the first time reported against enteroinvasive E. coli (HB. 101) and later also against Y. enterocolitica, S. aureus, Listeria monocytogenes, Pemphigus neonatorum, staphyloxanthin, Neisseria gonorrhoeae, Helicobacter pylori, Shigella dysenteriae, Bordetella pertussis, Y. pseudotuberculosis and S. pyogenes (24).
Lactoferrin has antiviral activity against a wide range of RNA and DNA viruses that infect humans and animals. Recent research suggests that lactoferrin on human respiratory virus Syncytial and prevents its effect. The latest studies on HIV showed that plasma proteins and lactoferrin found in milk had very strong activity against HIV (25).
Lactoferrin has been reported as an antiparasite drug in vitro. Amebiasis intestinal parasites cause diarrhea in children below five years of age and amebiasis is the fourth leading cause of death in the world. The recent results of the anti-parasitic activity of lactoferrin against intestinal amebiasis in the absence and presence of iron have been reported (26).
With regard to antimicrobial, antioxidant, antiviral, and antifungal activities of lactoferrin, instead of increasing the production of industrial antibacterial and chemical drugs, lactoferrin, mainly isolated from milk, can be introduced as a nutraceutical antibacterial agent.
Lactoferrin can reduce bacterial growth, as pointed out here and by others, and inhibit bacterial adhesion and biofilm formation; thus, it should be considered as a helpful antimicrobial therapeutic agent. Lactoferrin is able to bind iron, which is one of its important antibacterial features. Lactoferrin plays an important role in signal transduction, is anticancer, and has adhesive, immunomodulation, and antiviral activities. Regarding the increasing resistance to antibiotics, it is necessary to explore novel antimicrobial drugs to fight viral, bacterial and fungal diseases.