1. Background
Listeria monocytogenes is a species of bacteria, which causes the infection listeriosis (1), responsible for an estimated 1600 diseases and 260 deaths in the United States of America (USA) (2).
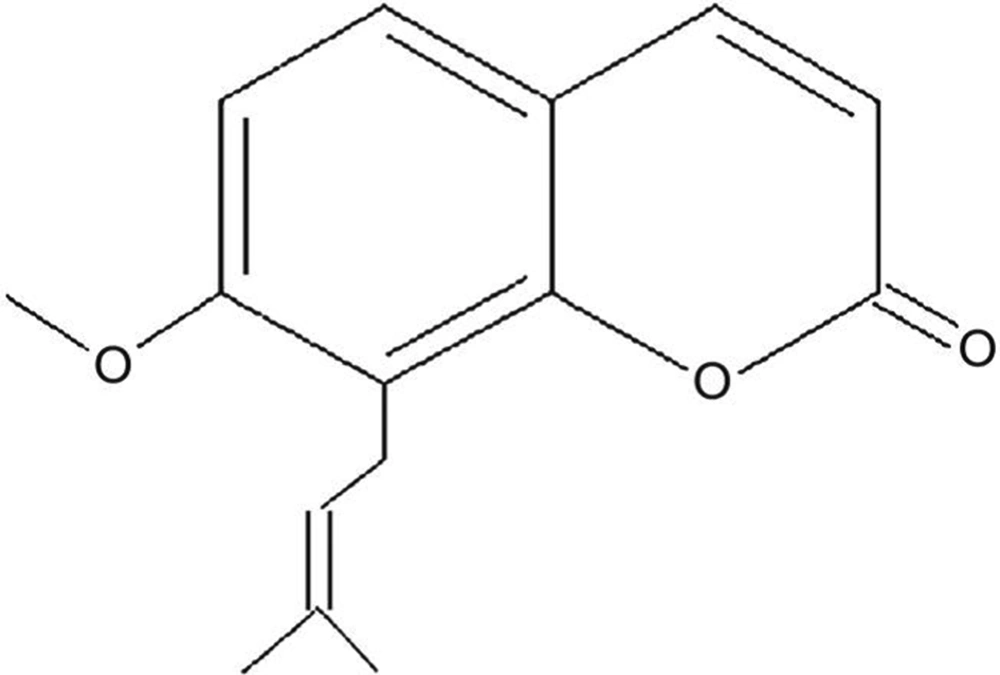
Plants can be useful sources of antimicrobial agents. Osthole (chemical formula shown in Figure 1) is isolated from several medicinal plants such as Cnidium monnieri. Osthole has antituberculous properties (3).
More than one mechanism can be involved in osthole activity. However, a pertinence of mechanisms may be discounted if the inhibition of energy generation occurs. These are because cells which cannot generate energy are unable to change their metabolism to adapt to the antimicrobial treatment.
2. Objectives
The aim of this study was to measure antibacterial activities and mechanisms of action of osthole.
3. Materials and Methods
3.1. The Antimicrobial Agent and Culture Conditions of L. monocytogenes
Osthole was supplied by Sigma-Aldrich. Listeria monocytogenes was grown on brain heart infusion (BHI) agar.
3.2. Determination of Bactericidal Concentrations of the Osthole
Concentrations of cultures were adjusted by dilution with the TSB + YE. Bactericidal concentrations of the osthole were determined as described by Shabala et al. (4).
3.3. Determination of ATP Level
The bacteria were grown to their growth phase as described above. The ATP level was determined as described by Shabala et al. (4).
3.4. ATP Analysis
ATP content of samples was measured by a light output reaction (5) in that light output was amplified by the DEAE-dextran (6).
3.5. Measurement of Protein Content
Protein content of cell suspensions was measured by utilization a modified Lowry method.
4. Results
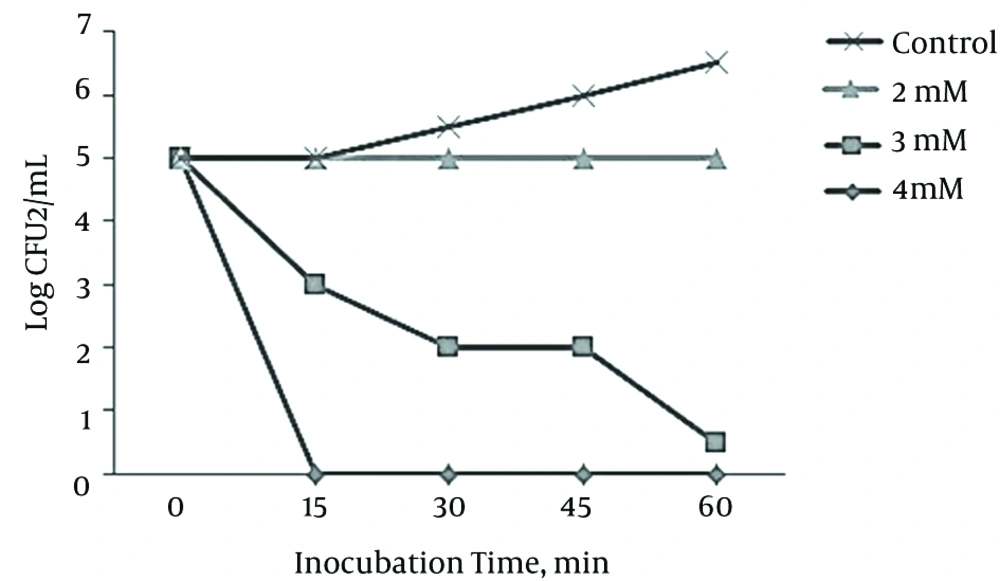
The experiment was carried out to measure the concentration of osthole required for the bactericidal activity against L. monocytogenes. Bactericidal effects were defined as a > 1-log depletion in the number of CFU in comparison with those of controls. Minimum concentration of the needed osthole for a bactericidal activity against L. monocytogenes was 2 mM (Figure 2).
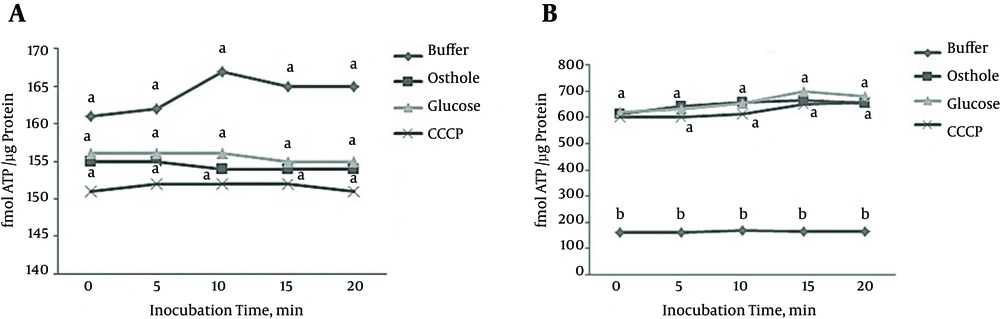
The suppressive effect on ATP generation: When glucose was supplied to L. monocytogenes cell in 25 mM HEPES, the ATP level was altered compared to the level in controls (Figure 3A). Incubation of L. monocytogenes with 2 mM osthole inhibited the uptake of ATP (Figure 3A). Furthermore 2 mM of osthole had no effects on ATP level when the energized cell was treated (Figure 3B).
(A) Cells were exposed to osthole at zero time, and all treatments except the buffer treatment were energized with 0.25% glucose for 5 minutes. (B) All treatments except the buffer treatment were energized with 0.25% glucose at zero time, and osthole was added at five minutes. The treatments included buffer, glucose, osthole (2 mM) and CCCP (10 µM). The data are the average of three experiments, and values, which are significantly different as determined by the Student t test (P = 0.05), are indicated by different letters.
5. Discussion
When applied to L. monocytogenes prior to glucose, it was observed that osthole inhibited the uptake in ATP level, which occurred in the controls. However, the osthole did not cause ATP reduction from the cell energized by glucose (7).
The current study findings are in contrast with the ion transport model for activities suggested by Ultee et al. (8) for the carvacrol. Carvacrol is bactericidal to Bacillus cereus at concentrations of 1.5 to 2 mM (9). Addition of 0.15 mM of carvacrol also disjoined membrane potential (10, 11). When the effects of carvacrol on the growth of B. cereus were compared with the effects of the related molecule, it was found which concentration of the molecules possessing the hydroxyl group were needed to the growth suppression (12). It is hard to differentiate between membrane disruptions and ion transport, and carvacrol is reported to uptake the staining of Pseudomonas aeruginosa (1, 13).
The CCCP may be anticipated in reducing ATP pools to preserve its normal pH; the bacteria utilize ATPase to export H+ (4, 14). The ATPase suppression is a real possibility as Rico-Munoz et al. (15) demonstrated which propyl gallate could reduce the activity of ATPase of S. aureus.
The L. monocytogenes has two glucose systems (16-18). It seems that osthole inactivates the transport mechanisms. The membrane effects cannot be decreased, as Walsh et al. (19) showed the potassium decline from E. coli treated with the eugenol.
5.1. Conclusions
The obtained results indicated the suppression of energy metabolisms of L. monocytogenes when the cell is treated with osthole.