1. Context
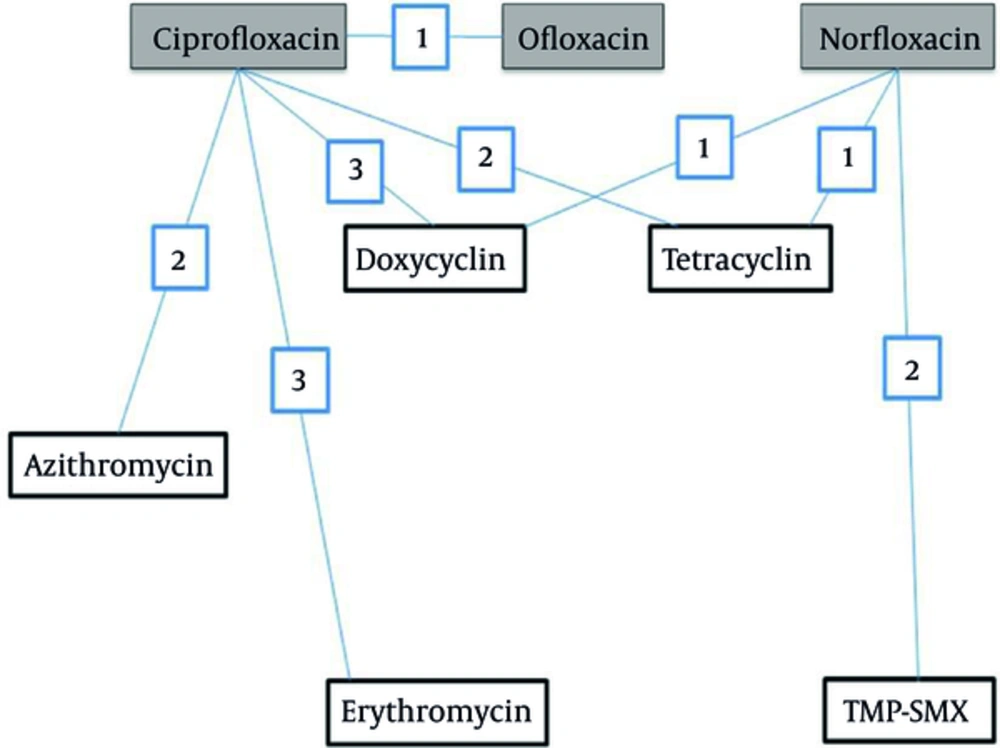
Rehydration therapy by appropriate oral or intravenous solutions is strongly advised during the treatment of cholera, a gastroenteritis disease with serious public health implications. Various international organizations engaged in promoting and implementing subjects relevant to public health infectious diseases recommend antimicrobial drug treatment for severely dehydrated patients with suspected cholera. Reduced duration of diarrhea and volume of stool and reduced duration of vibrio shedding due to adjunct treatment with antibiotic were reported in a recent Cochrane meta-analysis of several clinical trials conducted on a large sample of patients (1). Amongst various groups of antimicrobials, fluoroquinolones encountered significant emergence to treat gastroenteritis diseases. Evidence for various successes of clinical experience with quinolones in cholera and other enteric infections are reported in various international journals (2). A treatment network with quinolones elucidating various comparisons and the number of trials examining each comparison is presented in Figure 1. Ciprofloxacin, norfloxacin and ofloxacin were compared to placebo/no treatment (comparison not shown in Figure 1).
Utility of quinolone as adjunct in the treatment of cholera was first confirmed by norfloxacin as reported in studies first conducted in Calcutta, India (3), then in Lima, Peru (4), in Thailand (5), and in Salta, Argentina (6) respectively. Ciprofloxacin is another important quinolone with confirmed utility against gastroenteritis such as cholera and recorded as the most extensively studied amongst others. Excellent results with this fluoroquinolones was reported in an open, non-controlled study conducted by Doganci et al. (7), in two large randomized double-blind controlled studies conducted in Peru and Bangladesh (8), and in a large clinical trial conducted in Bangladesh (9). Results of these important studies clearly showed that quinolones were highly effective in all clinical parameters.
However, in recent years many studies from Africa, Asia and America reported the emergence of quinolone-resistant strains of V. cholerae (10, 11). Chander et al., and Saha et al., in different studies including 277 and 195 isolates of V. cholerae from Chandigarh (India) and Bangladesh respectively, both reported the emergence of ciprofloxacin-resistant strains of V. cholerae (12-14). In a study including strains of V. cholerae species isolated from 61 cases with gastroenteritis admitted to the gastroenteritis wards of Karnataka, Hubli (South India) and hospitalized for one to seven days during the cholera epidemic of 2002, Krishna et al. reported norfloxacin and ciprofloxacin resistance with the prevalence of 12.5% (15). A warning record on progressive increases in ciprofloxacin and norfloxacin resistance among V. cholerae O1 strains with the highest prevalence of 38.8% in 1999 and 25% in 2000, respectively was demonstrated by Garg et al., in a study on patients with cholera admitted to the Infectious Diseases Hospital, Calcutta, India (16). Mercy et al., in a study on V. cholerae O1 species isolated from different regions of Kenya from 2007 to 2010 reported resistance against nalidixic acid and ciprofloxacin with the prevalence of 89% and 2.3%, respectively (17). In a study aimed to determine the antibiotic susceptibility patterns of 118 strains of V. cholerae O1 isolated in 2010 at Jawaharlal Nehru institute of post graduate medical education and research, Puducherry, India, Mandal et al., reported resistance to ciprofloxacin with a modest occurrence of 4.2% (18). While a study by Eibach et al., reported that the increase in ciprofloxacin resistance reached about 98% in 2014 (19) (Table 1).
| Year | Number of Isolates | Number of Resistant Strains (%) | |||
|---|---|---|---|---|---|
| Nalidixic Acid | Ciprofloxacin | Norfloxacin | Ref. | ||
| 1989 | 49 | 3 (6.1) | ND | ND | (16) |
| 1990 | 59 | 1 (1.7) | ND | ND | (16) |
| 1991 | 30 | ND | ND | (16) | |
| 1992 | 26 | 2 (7.7) | (16) | ||
| 1993 | 20 | 1 (5) | (16) | ||
| 1994 | 74 | 73 (98.6) | (16) | ||
| 1995 | 84 | 82 (97.6) | 2 (2.4) | 3(3.6) | (16) |
| 1996 | 69 | 68 (98.5) | 4 (5.8) | (16) | |
| 1997 | 53 | 50 (94.3) | 10 (18.9) | 4 (7.5) | (16) |
| 1998 | 201 | 197 (98) | 20 (10) | 1 (0.5) | (16) |
| 1999 | 49 | 49 (100) | 19 (38.8) | 8 (16.3) | (16) |
| 2000 | 16 | 12 (75) | 3 (18.7) | 4 (25) | (16) |
| 2007-2010 | 44 | 39 (89) | 1 (2.3) | (17) | |
| 2010 | 118 | ND | 5 (4.2) | ND | (18) |
| 2014 | 92 | 62 (100) | 61 (98.4) | (19) | |
Vibrio cholerae O1 Species Isolates From Different Cholera Outbreaks and the Prevalence of Resistance to Quinolones
2. Evidence Acquisition
Results of the search in clinical trials.gov database and the cochrane database of systematic reviews by John Wiley & Sons, Ltd. were synthesized to illustrate and/or relate the reported clinical success and/or limits of quinolones as adjuncts to treat cholera. Medline, PubMed, Google and other databases were also used to select about 80 publications on cholera and quinolones resistant mechanisms in V. cholera to evaluate how the treatment of cholera with these drugs is evolved regarding the various quinolone-resistant V. cholerae strains.
3. Results
3.1. Quinolone Mechanisms of Activity
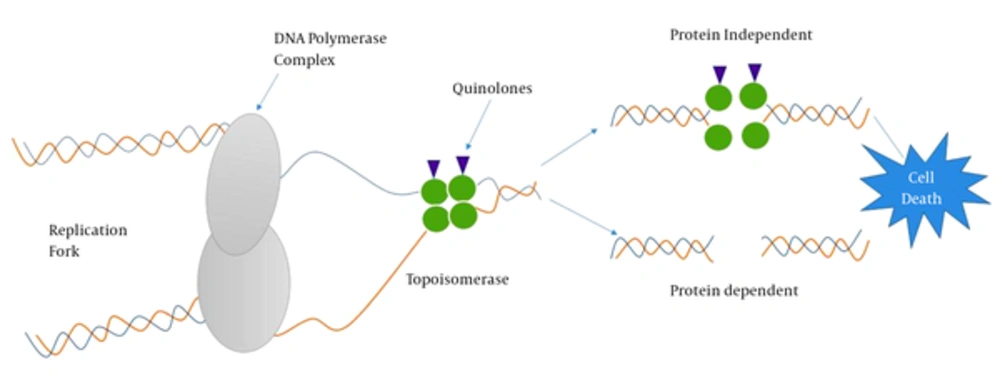
Understanding antibiotic resistance mechanisms requires an understanding of where antibiotics exert their effect. Cellular events such as DNA replication and mRNA transcription that take place during cell cycle or cell-division cycle require the intervention of topoisomerases such as DNA gyrase (type II topoisomerase) recognized to participate in relieving some of the stress during DNA synthesis (20). Thus, chemists and pharmacists involved in the synthesis of quinolones do exploit these conditions to target DNA-topoisomerase complexes (21, 22). Quinolones are bactericidal compounds that inhibit bacterial DNA from unwinding and duplicating through inhibition of the topoisomerase II ligase domain. This interference consequently leads to transitory DNA fragmentation and by catalyzing the passage of DNA segments within the cuts before closing them and though leading to rapid cell death (Figure 2). DNA gyrase, also referred as topoisomerase II, consists of two polypeptide subunits (GyrA and GyrB) with the function to regulate links via relaxing supercoils and by introducing both positive and negative supercoils between DNA double strands. The A subunit (encoded by gyrA gene) carries out binding of DNA, B subunit mediates the introduction of negative supercoils, and then A subunit participates in the reunion of DNA strands (23, 24). Topoisomerase IV, similar to topoisomerase II, consists of two polypeptide subunits (ParC and ParE) with the role to participate in separating new replicates of chromosomes following the replication process. Quinolones binds to the A subunit of topoisomerase II and though interfering in various steps involved in the DNA replication process.
3.2. Chromosomal Mediated Resistance Involving Mutations in Topoisomerase
In many bacteria, such as V. cholerae, quinolones resistance is a chromosomal mediated resistance involving mutations in the quinolone resistance-determining regions (QRDRs) of topoisomerases genes gyrA and parC. Quinolone resistance in V. cholerae species is mainly observed in strains harboring mutations in gyrA and parC genes of topoisomerase II and IV respectively (25, 26). Substitutions of single amino acid (Ser-83; Ile in gyrA and Ser-85; Leu in parC) were observed in genes of topoisomerase II and IV of V. cholerae O1 with marked reduced susceptibility to ciprofloxacin (27).
3.3. Efflux Resistance Mechanisms
The efflux system pumps out a variety of structurally and functionally distinct antimicrobials including quinolones from bacterial cells. To date, advancement in biomedicine research oriented through the understanding of the biochemistry of Gram-negative bacteria cell wall and the mechanisms involved in resisting antimicrobials such as quinolones significantly contributed to the discovery and characterization of various multidrug and drug-specific efflux pumps from bacteria.
Vibrio cholerae efflux pumps are categorized into two major families, such as the ATP-binding cassette (ABC) superfamily (28), and the proton-motive force (PMF) pump families. The PMF sub-families include the major facilitator superfamily (MFS) (29), the multidrug and toxic compound extrusion (MATE) family (30), the small multidrug resistance (SMR) family (31) and the resistance-nodulation-division (RND) superfamily (32-34). In V. cholerae, six putative genes from MATE family of efflux pumps are reported. Involvement of genes such as VcmB, VcmD, VcmH, VcmN, VcmA and VcrM (35) with energy dependent efflux toward fluoroquinolones is demonstrated in Escherichia coli and V. cholerae background. Recently, homologue of NorM in Vibrio parahaemolyticus (36) reported to mediate resistance to hydrophilic fluoroquinolones, aminoglycosides and norfloxacin was discovered in the genome of V. cholerae O1 El Tor N16961 (37). Among the V. cholerae efflux systems, MFS transporters and the PMF-dependent efflux were reported in separate studies to confer resistance to nalidixic acid (38) and various quinolones (39) in clinical strains of V. cholerae. RND efflux systems were encoded by six operons. Four operons (VexAB, VexCD, VexGH and VexIJK) out of six were reported to confer resistance to antimicrobials in V. cholerae (40-42). VexAB was associated with resistance to several antimicrobials determined by mutational analysis (40). In a recent study conducted by Jun O et al., inherently reduced expression of gene vca0421 in V. cholerae O1 was demonstrated to confer resistance to fluoroquinolones in V. cholerae O1 (43-47).
3.4. Plasmid Mediated Quinolone Resistance
Plasmids carrying genes that encode drug resistance determinants spread very fast in mixed bacteria communities. However, the wild strains of V. cholerae are mostly reported as poor hosts for plasmids carrying genes. The frequency of plasmid carriage in clinical strains of V. cholerae O1 was less (2%) compared with clinical non-O1 strains (25%) as reported elsewhere. Most of the clinical strains of V. cholerae O1 isolated from different geographical regions lacked the plasmids (48, 49), and when present, the size and functions of plasmids were not uniform. A study reported some of the naturally occurring conjugative and non-conjugative plasmids harboring drug resistance determinants in environmental and clinical isolates of V. cholerae encoding genes inducing various antibiotics resistance.
Conjugative plasmids evolved to increase the relative fitness of bacteria had implications both for maintenance and spread of plasmids. Transfer of multiple-drug-resistance from V. cholerae to E. coli (K12) was demonstrated in-vitro with conjugative plasmids. Transfer of multiple-drug-resistant (MDR) plasmids of group InC through conjugation with other bacteria was reported in a study conducted by Glass et al. This plasmid conferred resistance to a number of antibiotics including quinolones (50). MDR V. cholerae species isolated in Kenya harbored a 100 MDa plasmid of group IncC (51). In Algeria, the IncJ plasmids (IncJ incompatibility group) containing antibiotic resistance marker was identified for the first time in V. cholerae El Tor (52).
An emerging clinical concern that causes reduced susceptibility to quinolones was experienced with plasmids identified to carry quinolone resistant genes (53).Plasmid-quinolone resistant genes include intrinsic or qnr genes (22, 54, 55), reported to protect topoisomerases from quinolones in bacteria (56). Modest findings reported in a study on V. cholerae isolates in Brazil described qnrVC1, a homolog to qnr. Limitations regarding this study include lack of molecular mechanisms evidencing that qnrVC1 could be linked to reduced susceptibility of ciprofloxacin in V. cholerae (57). However, Hong Bin Kim et al., in a recent study on V. cholerae species isolated from Bangladesh, described a new qnr homolog, qnrVC3 and a large number of mutations in topoisomerase target enzymes that confer higher levels of transferable quinolone resistance (58, 59).
4. Conclusions
The application of antibiotics as adjunct to treat patients with cholera infection is encouraged by various international health organizations such as world health organization (WHO) and Medecin Sans Frontiere (MSF). Quinolones are one of the most important classes of antibiotics available to treat gastroenteritis including cholera. The emergence of multidrug or specific drug resistant V. cholerae strains significantly affected the clinical contribution of these drugs. To date, various studies reported comprehensive descriptions of various molecular mechanisms and mutations in topoisomerases enzymes involved in or causing drug resistance, respectively. Quinolones are in the clinics to treat cholera for several decades, but knowledge about the mechanisms of interaction between quinolone and its specific targets (i e, topoisomerases enzymes) and how mutations cause resistance is only in progress but not conclusive. Since the emergence of quinolones-resistant V. cholerae may significantly influence the strategies of controlling cholera, locally and regionally based surveillance information on antimicrobial susceptibility of V. cholerae should give importance to this aspect. Hopefully, this review can be used as a resource document to direct the development of new promising generation of quinolones to treat cholera based on better understanding of the molecular mechanisms of action of these synthetic drugs.