1. Background
Rotavirus-induced diarrhea (RD) is the leading cause of severe diarrhea in young children and infants (1). An estimated 2.3 million hospitalizations and approximately 527000 deaths in children aged < 5 years are caused by rotavirus annually worldwide (2). The diarrhea in human is associated with the dysfunction of intestinal mucosal cell absorption, resulting in the intestinal microecological imbalance and changes in composition of fecal metabolites (3, 4). Previous studies have revealed the changes in fecal microbiota of infants and children infected with RD (5, 6). However, reports on the differences in the fecal metabolites profile between the RD infants and healthy (H) infants are limited.
Among many fecal metabolites, the changes in fecal amino acids (AAs) and fatty acids (FAs) contributed to reveal the relationship between intestinal diseases and fecal metabolites profile (7, 8). On the one hand, fecal proteins profile has been reported to be affected by intestinal diseases, and the significant differences in composition of fecal proteins have been found in patients suffering from intestinal disease, such as a significant increase of calprotectin and lactoferrin in feces of patients with inflammatory bowel disease (9, 10). Meanwhile, as an important metabolite of protein metabolism, a range of fecal AAs levels were up-regulated or down-regulated due to the effects of intestinal diseases, which improved the understanding of changes in protein metabolism under the influence of intestinal diseases (11, 12). On the other hand, the composition of fecal FAs containing short-chain fatty acids (SCFAs), medium-chain fatty acids (MCFAs), and long-chain fatty acids (LCFAs) have also been altered due to inflammation or diarrhea (13-15). Huda-Faujan et al. (13) revealed that inflammatory bowel disease leads to the decrease of acetic acid, butyric acid and propionic acid, and increase of lactic and pyruvic acids in; feces. De et al. (14) found the levels of fecal MCFs of patients with inflammatory bowel disease decreased significantly, such as pentanoate, hexanoate, heptanoate, octanoate and nonanoate. Yoshioka et al. (15) reported that intestinal secretion induced by cholera toxin might delay the mucosal uptake and lymphatic transport of LCFs, and the amount of linoleic acid transported into the intestinal lymph was delayed and reduced in cholera toxin-treated rats. Therefore, in order to understand fully the pathological characteristics of RD infants, the changes in compositions of fecal proteins, AAs and FAs between RD infants and H infants should be elucidated.
In this study, fecal samples were collected from 15 RD infants and 15 H infants. All infants were given equal amounts of breast milk and complementary foods. The differences in proteins, AAs, and FAs profiles in fecal samples between RD infants and H infants were analyzed to deepen the understanding of relationship between RD and fecal metabolites profile.
2. Objectives
The thirty fecal samples used in this study were collected at a period from November 2018 to February 2019, among them, fifteen fecal samples were obtained from the RD infants (mean age 6.8 months old; range 4 - 9 months old; sex ratio 1:1), who were officially diagnosed as RD patients by the Harbin Children's Hospital using specific enzyme immunoassay method (6). Other 15 fecal samples were collected from the H infants ranging 4 to 9 months old (mean 6.8 months; sex ratio 1:1) were provided by volunteers in Harbin city. All infants were provided with the same diet, including equal amounts of breast milk, fruit puree, and rice flour. All fecal samples after collection were transferred into sterile plastic tubes, immediately dispatched to the laboratory where the study was conducted, and then stored at -80°C before use. In addition, this study was approved by the local ethics committee, and “freely given informed consent” was signed by parents of all infants.
3. Methods
3.1. Composition Assays of Fecal Proteins
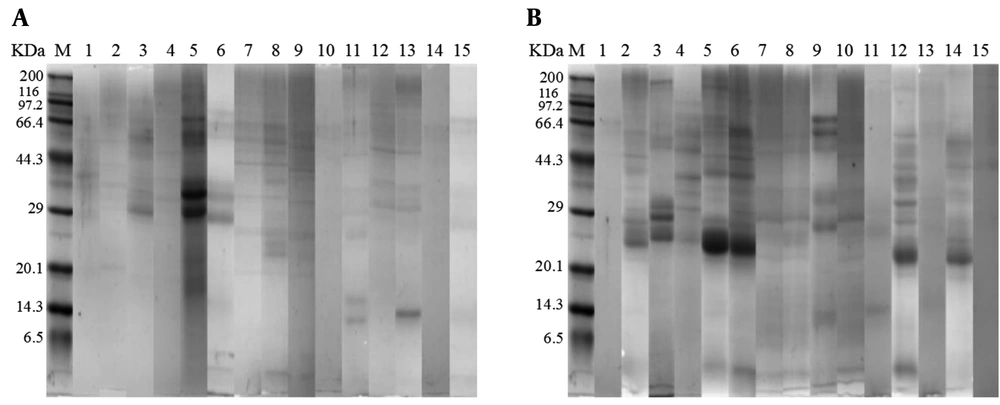
Approximately 100 mg fecal samples (wet weight) were transferred to a sterilized centrifuge tube, followed by the addition of trifluoracetic acid (0.15% v/v) of 200 μL and a gently mixing. All samples were centrifuged at 13,000 rpm for 5 min at 4°C using a refrigerating centrifuge (Heraeus, Hanau, Germany) to obtain fecal supernatants (16). The supernatants of 10 μL were heated at 95°C for 10 min, and analyzed with Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 5% stacking gel and 12% separating gel respectively and the Coomassie brilliant blue R-250C staining (17). The gels were scanned with an HP scanner (HP 1000, USA) to obtain corresponding images of protein bands. After that, the fecal protein feature was analyzed using a relative ratio analysis with Scion image PC software (Scion Co, Frederisk, USA) (18).
3.2. Assays of Fecal Amino Acids Profile
Fecal samples of 1 g (wet weight) were weighed and transferred to a hydrolysis tube. 6 mol/L HCl of 13 mL and 3 drops of phenol were added to the fecal samples followed by a vacuum treatment with 99.99% nitrogen. The hydrolysis tube was kept at 110°C for 22 h in a thermoelectric thermostat drying box to obtain the fecal hydrolysate. After filtration, fecal hydrolysate of 1 mL was dried with vacuum condition at 45°C, and then dissolved in 1mL of citrate buffer (pH 2.2). Finally, the types and contents of fecal AAs were determined with external standard method using an automatic amino acid analyzer (Hitachi, L-8900, Tokyo, Japan) (19).
3.3. Assays of Fecal Fatty Acids Profile
Approximately 100 mg (wet weight) fecal samples weighed and etherified with boron fluoride methanol methyl esterification method reported by Lópezlópez et al. (20). The supernatant containing methyl FAs was analyzed by high performance gas chromatography (HPGC) using Agilent 7890A gas chromatograph (Palo Alto, USA) to determine the relative amount of FAs in; feces.
3.4. Statistical Analysis
Each trial was independently carried out in triplicate. The data were analyzed by two-tailed Student's t-test to compare two groups using SPSS 20.0 software (SPSS, Inc., Chicago, IL, USA). The data were expressed as mean values ± standard deviations. The level of statistical significance was set at ≤ 0.05 for all analyses.
4. Results
The gel bands representing fecal proteins from RD infants and fifteen H infants are presented according to SDS-PAGE results (Figure 1). Using a relative ratio analysis by Scion image PC software, the relative proportion of each band was determined according to its brightness (Table 1). The results showed that all bands were divided into 17 groups based on the molecular weight of proteins, among them, significant differences (P < 0.05) in relative proportions of fecal proteins were found in four groups, including 50 - 55 KDa, 67 - 69 KDa, 79 - 80 KDa, and 84 - 85 KDa. In addition, compared to H infants, the relative proportions of fecal proteins with 50 - 55 KDa, 79 - 80 KDa, and 84 - 85 KDa from RD infants decreased significantly (P < 0.05), meanwhile, the relative proportion of fecal proteins with 67 - 69 KDa increased significantly (P < 0.05).
| Protein Molecular Weight (KDa) | Relative Proportion (%) | |
|---|---|---|
| RD Group | H Group | |
| 150 - 160 | 12.09 ± 2.45A | 14.23 ± 3.83A |
| 140 - 150 | 7.07 ± 4.27A | 5.03 ± 2.15A |
| 84 - 85 | 10.88 ± 5.38A | 0B |
| 79 - 80 | 7.70 ± 4.33A | 1.47 ± 0.25B |
| 67 - 69 | 0A | 8.25 ± 2.05B |
| 58 - 66 | 19.51 ± 5.72A | 11.67 ± 7.77A |
| 50 - 55 | 6.55 ± 1.77A | 0B |
| 43 - 44 | 6.20 ± 1.21A | 8.42 ± 2.12A |
| 42 - 43 | 11.46 ± 2.05A | 7.68 ± 1.34A |
| 39 - 41 | 17.19 ± 6.93A | 16.42 ± 4.46A |
| 37 - 39 | 10.02 ± 4.87A | 10.21 ± 4.00A |
| 35 - 37 | 6.55 ± 2.03A | 5.16 ± 1.64A |
| 33 - 35 | 6.60 ± 1.76A | 9.76 ± 2.65A |
| 30 - 31 | 8.75 ± 6.92A | 6.23 ± 3.82A |
| 22 - 26 | 22.60 ± 11.11A | 29.12 ± 11.94A |
| 19 - 20 | 14.35 ± 9.41A | 10.57 ± 4.34A |
| 14 - 16 | 10.48 ± 5.60A | 12.7 5± 0.30A |
Differences in Composition of Fecal Proteins Between RD Infants and H Infants a
Almost all sort of amino acids of infants; feces from RD and H samples were also assayed (Table 2). The results showed that RD infants; feces were detected to have decreased levels of the AAs (aspartic acid, threonine, serine, glutamic acid, glycinc, alanine, valine, methionine, isoleucine, leucine, lysine, phenylalanine, histidine, arginine, and proline) when compared to healthy subjects; feces (P < 0.05).
| Amino Acid Ingredient | Levels (mg/100 mg) | |
|---|---|---|
| RD Group | H Group | |
| Aspartic acid | 0.288 ± 0.158A | 0.561 ± 0.190B |
| Threonine | 0.244 ± 0.119A | 0.463 ± 0.174B |
| Serine | 0.208 ± 0.117A | 0.374 ± 0.133B |
| Glutamic acid | 0.457 ± 0.261A | 0.766 ± 0.260B |
| Glycinc | 0.149 ± 0.085A | 0.275 ± 0.098B |
| Alanine | 0.194 ± 0.106A | 0.380 ± 0.116B |
| Cystine | 0.147 ± 0.057A | 0.189 ± 0.063A |
| Valine | 0.271 ± 0.111A | 0.430 ± 0.108B |
| Methionine | 0.224 ± 0.134A | 0.440 ± 0.242B |
| Isoleucine | 0.131 ± 0.076A | 0.223 ± 0.079B |
| Leucine | 0.244 ± 0.137A | 0.421 ± 0.155B |
| Tyrosine | 0.097 ± 0.420A | 0.137 ± 0.077A |
| Phenylalanine | 0.179 ± 0.077A | 0.258 ± 0.082B |
| Lysine | 0.218 ± 0.169A | 0.354 ± 0.175B |
| Histidine | 0.087 ± 0.041A | 0.126 ± 0.047B |
| Arginine | 0.118 ± 0.073A | 0.254 ± 0.096B |
| Proline | 0.171 ± 0.078A | 0.288 ± 0.136B |
| Isoleucine | 0.131 ± 0.076A | 0.223 ± 0.079B |
| Leucine | 0.244 ± 0.137A | 0.421 ± 0.155B |
Differences in Levels of Fecal Amino Acids Between RD Infants and H Infants a
The compositions of fecal FAs of RD infants and H infants were analyzed. Compared to H infants, the relative proportions of butyric acid (C4:0), elaidic acid (C18:1N9T), linoleic acid (C18:2N6C), cis-11, 14-icotenic acid (C22:0), cis-11, 14, 17- epoxyeicosatrienoic acid (C20:3N3), cis-13, 16-docosanoic acid (C22:2), and cis-7, 10, 13, 16,19 docosapentaenoic acid (C20:5N3) in; feces from RD infants decreased significantly (P < 0.05). The significant increases in the relative proportions of caprylic acid (C8:0), decanoic acid (C10:0), undecanoic acid (C11:0), lauric acid (C12:0), tridecanoic acid (C13:0), myristic acid (C14:0), myristoleic acid (C14:0), palmitic acid (C16:0), cis-10-heptadecaenoic acid (C17:1), oleic acid (C18:1N9C), and γ-linoleic acid (C18:3N6) were found in the; feces collected from RD infants (P < 0.05) (Table 3).
| Fatty Acid Ingredient | Relative Proportion (%) | |
|---|---|---|
| RD Group | H Group | |
| Butyric acid (C4:0) | 6.322 ± 1.101A | 9.241 ± 1.950B |
| Caprylic acid (C8:0) | 0.753 ± 0.106A | 0B |
| Decanoic acid (C10:0) | 0.691 ± 0.150A | 0.244 ± 0.118B |
| Undecanoic acid (C11:0) | 0.380 ± 0.084A | 0B |
| Lauric acid (C12:0) | 6.431 ± 1.701A | 1.336 ± 0.142B |
| Tridecanoic acid (C13:0) | 0.340 ± 0.106A | 0B |
| Myristic acid (C14:0) | 11.862 ± 7.845A | 3.783 ± 0.674B |
| Myristoleic acid (C14:1) | 0.331 ± 0.177A | 0B |
| Pentadecanoic acid (C15:0) | 0.837 ± 0.310A | 0.893 ± 0.327A |
| Palmitic acid (C16:0) | 32.727 ± 3.219A | 19.596 ± 3.150B |
| Palmitoleic acid C (16:1) | 1.293 ± 0.504A | 1.191 ± 0.555A |
| Heptadecanoic acid (C17:0) | 1.255 ± 0.971A | 1.739 ± 0.686A |
| Cis-10-heptadecaenoic acid (C17:1) | 0.355 ± 0.313A | 0B |
| Stearic acid (C18:0) | 23.598 ± 5.804A | 20.369 ± 5.954A |
| Elaidic acid (C18:1N9T) | 0.228 ± 0.067A | 7.63 ± 2.076B |
| Oleic acid (C18:1N9C) | 21.468 ± 5.080A | 11.977 ± 4.783B |
| Linoleic acid (C18:2N6C) | 14.45 ± 9.581A | 29.798 ± 22.075B |
| Arachidic acid (C20:0) | 1.612 ± 0.487A | 1.114 ± 0.279A |
| γ-linoleic acid (C18:3N6) | 0.058 ± 0.031A | 0B |
| Cis-11-ethylenic acid (C20:1) | 1.527 ± 0.441A | 1.249 ± 0.998A |
| α-linoleic acid (C18:3N3) | 1.592 ± 0.322A | 1.348 ± 0.752A |
| Heneicosanoic acid (C21:0) | 0.321 ± 0.105A | 0.338 ± 0.205A |
| Cis-11,14-icotenic acid (C20:2) | 0.658 ± 0.251A | 3.023 ± 0.929B |
| Behenic acid (C22:0 | 1.034 ± 0.872A | 0.955 ± 0.273A |
| Cis-8,11,14-epoxyeicosatrienoic acids (C23:6) | 0.437 ± 0.396A | 0.598 ± 0.386A |
| Erucic acid (C22:1N9) | 0.511 ± 0.171A | 0.956 ± 0.333A |
| Cis-11,14,17-epoxyeicosatrienoic acids (C20:3N3) | 0A | 0.602 ± 0.367B |
| Arachidonic acid (C20:4N6) | 2.235 ± 2.013A | 3.177 ± 0.884A |
| Cis-13, 16- docosanoic acid (C22:2) | 0A | 2.294 ± 0.798B |
| Lignoceric acid (C24:0) | 0.625 ± 0.473A | 1.06 ± 0.055A |
| Cis-7,10,13,16,19-docosapentaenoic acid (C20:5N3) | 0A | 7.068 ± 4.510B |
| Nervonic acid C24:1 | 0.362 ± 0.220A | 0.454 ± 0.362A |
| Cis-4,7,10,13,16,19-docosahexaenoic acid (C22:6N3) | 0.918 ± 0.881A | 1.041 ± 0.506A |
Differences in Composition of Fecal Fatty Acids Between RD Infants and H Infants a
5. Discussion
Protein metabolism could be affected by the intestinal mucosal damage caused by microbial dysbiosis, diarrhea and other intestinal deseases, leading to the changes in composition of proteins in feces (16, 21). Among them, alpha-1-antitrypsin in human milk could withstand the breakdown of digestive juices, and help the survival of other proteins especially during bacterial diarrhea (22). Increased fecal lactoferrin can be a significant indicator for monitoring intestinal inflammation in children with non-virus diarrhea (23). In addition, serum albumin was identified as a marker in the colorectal cancer, meanwhile, polymeric-immunoglobulin receptor could not protect rotavirus from expanding in the gut (16, 24). Similarly, after infants suffered from RD, the relative proportions of fecal proteins with 50-55 KDa, 79-80 KDa, and 84 - 85 KDa decreased significantly (P < 0.05), meanwhile, the relative proportion of fecal proteins with 67 - 69 KDa increased significantly (P < 0.05). Given that all fecal proteins from infants in the study were derived from breast milk, it was reasonable to infer these differential proteins should correspond to polymeric immunoglobulin receptor (84 KDa), lactoferrin (80 KDa), serum albumin (69 KDa) and alpha-1-antitrypsin (53 KDa) according to the molecular weight of the proteins (10, 22, 25, 26). However, a deeper and more comprehensive study on the relationship between differential proteins and rotavirus infection is still needed in future.
As the structural units for proteins and polypeptide, AAs were participated in synthesis of bioactive molecules which played the key role in the regulation of signaling pathways and metabolism (27). AAs metabolism in complex gut environment provides a strategy for bacteria survival and growth with both positive and negative effects on host (28). For example, Marchesi et al reported the AAs degradation of inflammatory bowel disease (IBD) patients were promoted due to an impairment of the metabolic activity of the gut bacteria (29), meanwhile, De et al found a higher AAs level in the; feces of IBD patients, and considered it was because that inflammatory conditions induce large energy requirements to repair the damaged mucosa leading to enhanced protein catabolism (30). Besides, Bjerrum et al revealed that the levels of aspartic acid and glutamate in fecal extracts from inactive Crohn’s disease (CD) patients were significantly reduced compared with those of healthy samples (11). Our results showed that there were significant decreases in AAs levels in; feces of RD infants, including aspartic acid, threonine, serine, glutamic acid, glycinc, alanine, valine, methionine, isoleucine, leucine, phenylalanine, lysine, histidine, arginine, and proline, which suggested a reduced level of AAs in; feces linked to the diarrhea induced by rotavirus defection.
In previous studies, as the significant metabolite of intestinal microbiota, the changes in levels of SCFAs in; feces of diarrhea patients have been a focus of research (31, 32). However, intestinal diseases can not only change the diversity of intestinal microbiota, but also cause intestinal metabolic disorders. Therefore, it is necessary to conduct a comprehensive analysis on the differences in fecal FAs profile consisting of SCFAs, MCFAs and LCFAs between RD infants and H infants.
Butyric acid was considered as one of the most important SCFAs associated with human health, and could be produced by metabolism of intestinal microorganisms; therefore, the differences in biodiversity of fecal microbiota can explain the possible reason for butyric acid alteration of patients with intestinal diseases (33). Some researchers suggested that the lower levels of butyrate and propionate in; feces of CD and ulcerative colitis (UC) diarrhea patients should be the consequence of an inflammation-driven intestinal dysbiosis (11). Further, De et al found the depletion of butyric acid in feces of IBD patients was linked to a shift in the composition and metabolic activity of colonic microbiota (30). In this study, the level of butyric acid in; feces of infants decreased significantly after infection with rotavirus, which was similar to the study reported by Canani et al. who found a significant reduction in the concentration of fecal butyric acid of patients with intestinal diseases (34). However, in our previous report, we detected formic acid, acetic acid, propionic acid and butyric acid in the feces of RD and H infants who were between 0 and 6 months old, among them, acetic acid was the dominant SCFA (35), while, in current study, only butyric acid was detected in the feces of RD and H infants who were between 4 and 9 months old. In addition, there was no significant difference in the levels of these five SCFAs in the feces between 0-6-month-old infants with and without RD (35), on the contrary, the level of butyric acid in RD infants was significantly reduced compared to H infants in this study. Since the infants in the two studies had the same diet, the difference in SCFAs should be due to the age, in particular, the intestinal flora of infants younger than 1 year old is a dynamic colonization, which suggests that a more detailed age division should be made in the clinical analysis of RD infants in order to implement specific treatment measures for patients of different ages.
In prior study, MCFAs were found to be significantly reduced in the; feces of patients with CD, UC and pouchitis, and were used as a class of metabolic biomarkers of disease-related changes (36). Garner et al also found a lower prevalence of some MCFs in patients with UC than that in asymptomatic individuals (37). Similarly, in this study, the significant decreases in relative proportions of MCFAs and LCFAs, such as C18:1N9T, C18:2N6C, C22:0, C20:3N3, C22:2, and C20:5N3 in; feces from RD infants were found in; feces of RD infants. Furthermore, the relative proportions of C8:0, C10:0, C11:0, C12:0, C13:0, C14:1, C14:0, C16:0, C17:1, C18:1N9C, and C18:3N6 were increased in the; feces from RD infants, which indicated that RD may affect the absorption of the above fatty acids. Thus, in addition to SCFAs, the metabolic profiles of MCFAs and LCFAs should also be analyzed as an important prospective longitudinal study.
In summary, we provided a comprehensive analysis to illustrate the effects of RD on protein, AAs and fat metabolism. The data of this study revealed the differences in compositions of fecal proteins, AAs and FAs (SCFAs, MCFs, and LCFs) between RD infants and H infants. These findings revealed the pathological characteristics of RD in infants, and expanded the understanding on relationship between RD and fecal metabolites profile.