1. Background
Valvular pulmonary stenosis (VPS) is a common congenital heart defect which is characterized by obstruction of right ventricle to the pulmonary arteries at the level of pulmonary valve. It is usually diagnosed based on a cardiac examination and is confirmed by echocardiography. VPS classified as mild to severe grading and critical neonatal cases which can be life-threatening due to inadequate antegrade pulmonary flow through the right ventricular outflow tract, and in this situation, survival is dependent on patency of ductus arteriosus (1).
This malformation was described by John Baptist Morgagni at 1761, but balloon pulmonary valvuloplasty (BPV) was initiated in 1982 by McCrindle and Kan. Since that time BPV was the procedure of choice with same effectiveness as a surgical correction but less invasive (2-5).
VPS is a common congenital heart defect and occurs in 0.6 to 0.8 per 1000 live births. However, the incidence may be underestimated as mild pulmonary stenosis may be considered a trivial lesion and not be referred to a pediatric cardiology center (6).
In this study, we evaluated the immediate results of balloon valvuloplasty and heart remodeling in midterm follow up.
2. Methods
We enrolled all patients with pure VPS who underwent valvuloplasty in pediatric catheterization laboratories of Faghihi, Namazi and Kowsar hospitals in Shiraz University of Medical Sciences from 2010 through 2014.
Echocardiography was performed with a Mindray DC 7 (Shanghai, China) using transducer 2 - 7 Mhz. Echocardiographic studies were done by one pediatric cardiologist. We evaluated several parameters of cardiac function to raise intra-observer reliability. Echocardiographic measurements were 2-dimentional, M-mode, Doppler, and tissue Doppler echocardiography. M-mode parameters consisted of the inter-ventricular septum, and left ventricular posterior wall diameter in systole and diastole while we measured ejection fraction and fractional shortening in long-axis view. We measured early diastolic inflow velocity (E), velocity of atrial contraction (A), and E/A ratio by Doppler echocardiography. Also tissue Doppler velocity was assessed in apical four-chamber view from lateral mitral and tricuspid valves annulus and the inter-ventricular septum. All values were compared with normal values from pediatric and fetal Z score calculator web site.
Initial success was considered as the immediate effect; while peak instantaneous pulmonary valve pressure gradient and right and left ventricular function were considered as the midterm outcome.
Patients under 18 years of age who had pure valvular pulmonary stenosis were enrolled in this study.
Balloon dilation was performed after informed consent was obtained from the patients’ parents. Catheterization and angioplasty were carried out under fluoroscopy guidance in the cardiac catheterization laboratory. The procedure was performed under conscious sedation. Antegrade femoral vein approach was used in all but three patients in whom jugular, axillary or umbilical vein approach was used. Indication for BPV was defined as moderate pulmonary valve stenosis with peak to peak gradient more than 40 mmHg or right ventricular pressure at least more than half of systemic ventricular pressure. The balloon size was 1.2 - 1.4 times the pulmonary valve annular diameter. The balloons were inflated at the site of pulmonary stenosis 2 - 3 times.
All echocardiographic measurements were performed in three heart cycles and mean value was used for analysis. All values were compared with normal values from calculator pediatric Z score web site (7, 8).
Our institutional ethics committee approved the research and we have not been supported by any funders.
2.1. Statistical Analysis
Data were analyzed using SPSS ver .20 (SPSS Inc., Chicago, IL, USA). Values were classified as mean ± standard deviation (SD) for the quantitative and percentages for other categorical variables.
Pearson correlation was used to evaluate the correlation between parameters. P-value <0.05 was considered as statistically significant.
3. Results
Among 104 patients with diagnosis of pure VPS who were evaluated after BPV, 93 cases had a successful procedure without any complications. Two cases had cardiac arrest that recovered with hydration and cardiac massage and 6 cases faced further complications (detachment of hydrophilic wire cover, mild pericardial effusion, sepsis, supra ventricular tachycardia, umbilical vein dissection) and BPV failed in 3 patients.
Median age of the patients was 1.2 years (3 days to 14 years) and weight 11.32 ± 7.7 kg at the time of balloon angioplasty. The patients had mean age of 4.48±3.71 on follow up, mean weight of 14.34 ± 8.49, and duration of follow up was 2.0 ± 1.87 years.
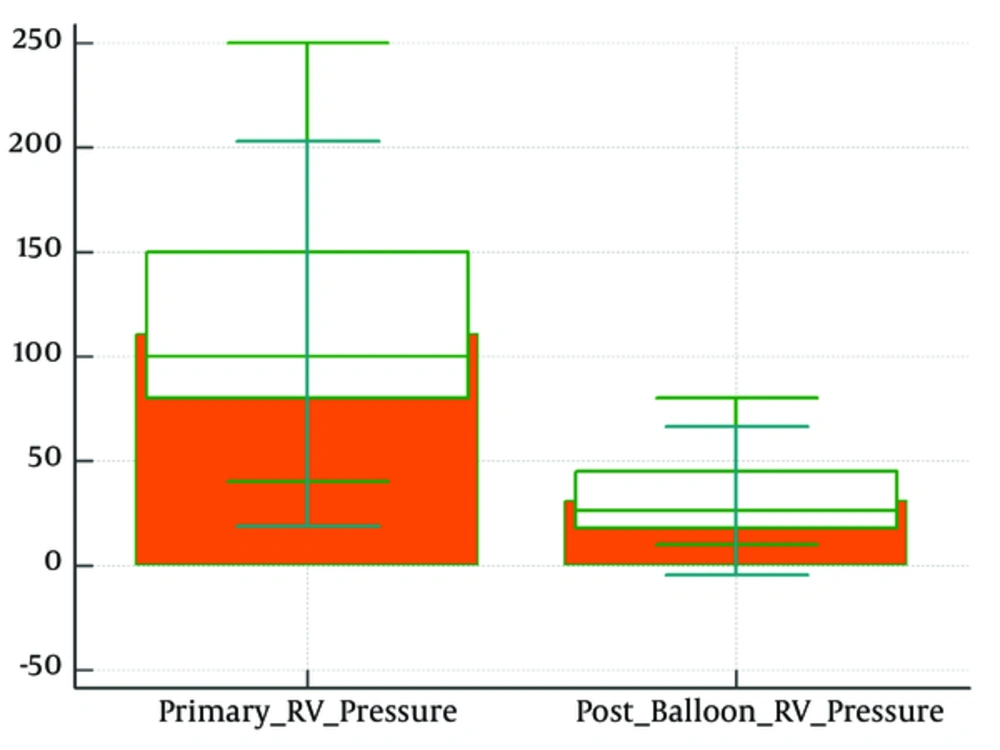
Mean peak right ventricular pressure was 110.84 ± 28.93 mmHg that decreased to 41.24 ± 19.11 mmHg (Figure 1).
Mean pulmonary artery pressure was 15.15 ± 4.86 mmHg that increased to 18.45 ± 7.18 mmHg. Mean peak to peak VPS gradient before balloon was 93.21 (± 33.34) that decreased immediately to 27.34 (± 14.91) after BPV.
Mild pulmonary insufficiency (PI) was detected in 72%, moderate PI in 26% and severe PI in 2%. All the patients with severe PI were neonates.
Two patients with dysplastic pulmonary valve who didn’t respond to BPV were referred for surgical correction, one of them had Noonan syndrome (Table 1).
| Cases | Age at Balloon, y | Weight at Balloon, kg | Pre balloon Pulmonary Stenosis Pressure Gradient, mmHg |
|---|---|---|---|
| 1 | 3 | 14 | 150 |
| 2 | 0.01 | 3 | 98 |
| 3 | 0.1 | 2.8 | 110 |
| 4 | 0.8 | 7 | 200 |
| 5 | 0.3 | 5.4 | 94 |
| 6 | 1.2 | 7.2 | 97 |
| 7 | 1.3 | 7.2 | 100 |
| 8 | 0.1 | 2 | 100 |
M-mode echocardiographic evaluation of patients during follow up showed that left ventricular systolic and diastolic diameters were within normal values for age but septal hypertrophy and decreased left ventricular dimensions were detected in a significant number of the patients (Tables 2 and 3).
| Variable | Mean ± SD | Mean Z-Score | Percent with Z Score > 2 | Percent with Z Score < -2 |
|---|---|---|---|---|
| Interventricular septal thickness in diastole, cm | 0.62 ± 0.17 | 0.96 | 22.4 | 0 |
| Left ventricular end-diastolic dimension, cm | 2.69 ± 0.66 | -1.15 | 0 | 23.5 |
| Posterior wall thickness in diastole | 0.59 ± 0.16 | 1.27 | 10.7 | 0 |
| Interventricular septal thickness in systole, cm | 0.78 ± 0.21 | 0.69 | 13.9 | 0 |
| Left ventricular end-systolic dimension, cm | 1.6 ± 0.55 | -1.78 | 0 | 44.4 |
| Posterior wall thickness in systole, cm | 0.68 ± 0.16 | -1.04 | 0 | 10 |
| Ejection fraction, % | 77.81 ± 7.75 | - | ||
| Shortening fraction, % | 43.24 ± 8.15 | - |
| Variable | Mean ± SD | Z-Score | Percent with Z-Score > 2 | Percent with Z-Score < -2 |
|---|---|---|---|---|
| ET | 90.08 ± 38.16 | 2.16 | 44 | 0 |
| AT | 69.54 ± 21.53 | 1.79 | 44 | 0 |
| E/A ratio | 1.28 ± 0.56 | -0.06 | 13 | 0 |
Abbreviations: AT, Peak A velocity flow in tricuspid valve; E/A ratio, ratio of ET to AT; ET, Peak E flow velocity in tricuspid valve.
Tissue Doppler evaluation showed decreased E velocity and increased A velocity of tricuspid valve (Table 4). Z-score of E to Ea ratio of tricuspid valve was more than normal.
| Variable | Mean ± SD | Mean Z-Score | Percent with Z-Score > 2 | Percent with Z-Score < -2 |
|---|---|---|---|---|
| Ss | 8.75 ± 2.26 | 1.21 | 26 | 2.3 |
| Eas | 10.6 ± 2.92 | -0.5 | 0 | 16.3 |
| Aas | 9.81 ± 2.66 | 2.58 | 53 | 0 |
| St | 12.91 ± 2.85 | 0.41 | 12 | 6 |
| Eat | 14.19 ± 4.58 | -0.63 | 0 | 8.7 |
| Aat | 14.98 ± 4.78 | 1.75 | 46 | 4.2 |
| Et/Eat | 7.09 ± 5.09 | 2.99 | 59 | 0 |
Abbreviations: Aas, peak late diastolic annular velocity; Aat, peak late diastolic lateral annular velocity; Eas, peak early diastolic annular velocity; Eat, peak early diastolic lateral annular velocity; Et/Eat, E Doppler flow on Eat tissue Doppler flow; Ss, peak systolic annular velocity; St, peak systolic lateral velocity.
Pulmonary artery size measurements showed normal range for body surface area (Table 5).
| Index | Mean of Index | Mean Z-Score | Percent with Z Score > 2 | Percent with Z Score < -2 |
|---|---|---|---|---|
| MPA, cm | 1.27 ± 0.67 | -0.54 | 10.5 | 10.5 |
| RPA, cm | 0.82 ± 0.37 | 0.52 | 38 | 18.8 |
| LPA, cm | 0.62 ± 0.16 | -0.1 | 21.6 | 14.3 |
Abbreviations: APA, right pulmonary artery; LPA, left pulmonary artery; MPA, main pulmonary artery.
4. Discussion
The pulmonary valve dilatation by a percutaneous balloon is the method of choice for treatment of pulmonary valve stenosis in children. BPV is an effective and safe method, with low mortality and morbidity (9, 10). BPV was developed as an alternative method to surgical valvotomy in 1948 by Brock (11).
From the point of view of decreasing pressure gradient, in our study, mean right ventricular pressure was 110.84 ± 28.93 that decreased to 41.24 ± 19.11. In a study by Li H et.al peak right ventricular pressure gradient declined from 112.0 ± 21.0 to 50.4 ± 15.9 mmHg (P < 0.001), and mean instantaneous pulmonary valve gradient in follow up was 29.88 ± 12.44 obtained by echocardiography (12).
In an investigation on 52 patients by Mahnert et.al. with BPV, the mean peak to peak pulmonary valve gradient declined from 79.9 ± 37.3 to 37.2 ± 29.6 mmHg after the procedure, while residual gradient more than 36 mmHg persisted in 19 patients and after 2 years, the gradient decreased to less than 36 mmHg in 10 out of 19 (52.63%) patients (13).
In the study of Petersen et al. the mean peak to peak pulmonary valve pressure gradient dropped from 66.2 ± 21.4 to 21.5 ± 15.9 mmHg after BPV (14).
Gupta D et al. followed 62 patients 9 months to 44 years of age for 1 to 10 years (mean 6.4 years) after BPV and found that the mean peak to peak transvalvar gradient fell from 93 to 19 mmHg immediately after the procedure, whereas instantaneous pressure gradient was 18 mmHg at follow-up (15).
Restenosis occurred in 4 (4.3%) of our patients who needed redilation of pulmonary valve and 3 (2.8%) cases needed surgery due to dysplastic pulmonary valve because they didn’t respond to BPV. Similar findings were noted in a series of 85 patients followed for up to 10 years. Repeated balloon dilation was required in 11 percent and surgical intervention for subvalvular or supravalvular stenosis in 5 percent (16). In another study, one (2.1%) case had restenosis that improved by redilation (17).
There were some complications among our patients. Two cases had cardiac arrest that recovered with hydration and cardiac massage and 6 cases developed other complications (detachment of the cover of hydrophilic catheter, mild pericardial effusion, sepsis, SVT, umbilical vein dissection), and 3 BPVs failed. Most complications were in patients less than one year old. So incidence of the complications is low. The complications increase when the BPV is performed in the neonatal period (17).
Also mortality rate of 0.2 percent and major complications of 0.6 percent were reported (15). Usually acute complications are generally minor and include a vagal response, catheter induced ventricular ectopy, right bundle branch block, and transient or permanent high grade AV nodal block (1, 16). Other complications include pulmonary valve regurgitation, tricuspid regurgitation, stroke, syncope, pulmonary artery rupture, pulmonary edema, cardiac perforation, and tamponade. In one study, acute complications occurred in 4.23% patients and in 1 patient dissection of the inferior vena cava occurred without retroperitoneal bleeding or hematoma. In this study two patients had convulsion during the procedure and also atrial fibrillation happened in 2 patients and one patient developed deep venous thrombosis in the right lower extremity that had undergone the procedure (17).
A further complication that may occur is PI. In our study 72% of the patients had mild, 26% moderate and 2% severe PI but no patient needed valve replacement on follow up. In a published study, mild PI occurred in 69.2%, and moderate PI in 30.8% (18).
Several researches have reported a high prevalence of PI after BPV, but no one required surgical intervention for therapy (19, 20) with the exception of Berman and colleagues, who reported that 6 of 107 BPVs needed pulmonary valve replacement due to right ventricular dilation (20). Careful selection of balloon size reduces the PI risk (21).
Another important complication is tricuspid valve insufficiency (TI). Moderate TI was detected in 1% of our patients just like in other researches (14-22).
M-mode echocardiographic evaluation of our patients showed normal left ventricular ejection fraction and normal Z-score of left ventricular end systolic and diastolic dimensions, normal free wall thickness whereas 20% of patients had septal hypertrophy during follow up.
In Doppler study of tricuspid valve 34% of patients had E/A ratio less than 1 and in 11% it was more than 2 which is an index of diastolic abnormality (22). Tissue Doppler evaluation showed significantly increased Aa velocity of septum and tricuspid valve. Also E/Ea tricuspid ratio had a significant difference with normal reference value for age.
A study by Vermilion on 14 patients before and after BPV showed that before BPV, the patients had higher peak A velocity (0.64 ± 0.28) versus control group (0.39 ± 0.08 m/s), and so lower E/A velocity ratio than normal subjects. But before and after BPV, there was no change in any Doppler index (23). Thus, patients with VPS have abnormal diastolic filling with filling velocity in early diastole and high filling velocity during atrial contraction. These abnormalities do not change early after successful BPV, suggesting that hypertrophy rather than afterload is the main factor for the impaired relaxation (23).
In the study by Saiki, before balloon valvuloplasty, the patients had a higher ejection fraction and smaller LV dimension than the control group. Also, before BPV, the patients had thick right ventricular wall and thick interventricular septum. These abnormalities did not change immediately after BPV or in short term after that, although they became normal during an intermediate term. Also before BPV, the patients had abnormal relaxation but immediately and at the short term period, there were no significant changes in the diastolic indices of the patients, later the abnormal indices became normal with improvement in the right ventricular wall thickness and septal hypertrophy (24).
4.1. Limitation of the Study
Retrospective collection of data was a limitation of the study.
4.2. Conclusions
BPV is a safe and effective treatment option for VPS children with low complication and mortality while right ventricular diastolic dysfunction may remain for a long term.