1. Context
Vesicoureteral reflux (VUR) is one of the most common anomalies of the urinary tract among children and can lead to progressive kidney injury and consequently kidney failure. In the long term, it is one of the main causes of high blood pressure, growth disorder, and renal failure in childhood. Endoscopic modification has been presented for vesicoureteral reflux treatment from the early 1980s and appeared as the foremost management in all grades of VUR (1, 2). This method has some positive characteristics like less length of treatment, lower charge, and is a less complex process than open surgery (3). These points of interest are significant in choosing a treatment strategy because the decision is usually based on the balance between the intervention’s side effects and the indistinctness of self-correction. Reducing problems and morbidities can increase the tendency toward endoscopic treatment in a selected group of patients (4). The success rate of this method is closely related to the VUR development stage and agent used (5). Morbidities following this procedure are not frequent and mainly include the ureterovesical junction obstruction and the growth of new contralateral reflux following treatment of unilateral VUR (6). Different materials such as polytetrafluoroethylene, polydimethylsiloxane, and bovine collagen have been used for the endoscopic suburethral application, and their cure rate has been different from 60 to 80 percent (7-9). However, they had documented side effects in some studies such as granuloma formation (10, 11), migration to distant organs (12), autoimmune reactions, and early recurrence of VUR (9). Also, new alternative agents like Urocol that certainly had a very effective role in improving the condition of these children has some hindrances. It has inadequate viscosity and sometimes it is stuck in the injection equipment and the procedure needs to be repeated, which means that the child should undergo another general anesthesia, cystoscopy, and intervention (13). Dextranomer/hyaluronic acid copolymer (Deflux®) as an artificial material, helps to grow collagen and fibroblasts and its hyaluronic acid can keep the implant in place. The complication of Deflux® includes activation of the immune reaction and the formation of granuloma pseudocysts around the Deflux® after the injection of this substance (14).
Nowadays, the extracellular matrix (ECM) is widely used in various forms in cellular and tissue engineering (15-17). Different types of ECM-based materials or tissue structures are developed for regeneration purposes. In this regard, using a natural scaffold (especially autologous) for infants and children, which has fewer complications, has attracted much attention. We aimed to summarize the worldwide experience with the various bulking agents used for endoscopic treatment of VUR, particularly the cure and complication rates. Also, we have discussed our newly introduced bulking agent: acellular lyophilized prepuce as a natural scaffold.
2. Evidence Acquisition
2.1. Dextranomer/Hyaluronic Acid Copolymer
Dextranomer/hyaluronic acid copolymer known as Deflux® is the most frequently used material for endoscopic treatment of VUR. It is composed of dextranomer microspheres in sodium hyaluronate solution (18). The success rate with Deflux® is inversely proportional to the grade of reflux. It is reported from 78.5% for grades I and II to 51% for grade V (19). Taskinlar et al. (20) reported the lowest success rate of 52.6% after the first injection that improved to 66.6% after the second injection. They retrospectively investigated children with VUR grade II-IV. The highest success rate of 98.9% is reported by Kajbafzadeh and Tourchi (21), when they used concomitant autologous blood and dextranomer/hyaluronic acid copolymer using hydrodistention autologous blood injection technique (HABIT) protocol for grades II-IV. Others reported the success rate between these two measures (52.6% - 98.9%) (22-27). Pichler et al. (22) reported post-operative UTI with persistent VUR in 2.25%. Taskinlar et al. (20) encountered UTI in 20% of their cases which is considerable.
Some complications have also been reported for Dx/HA. In addition to persistent VUR, obstruction is the most frequent complication, with an incidence ratio of 0% - 5.7% in published case series (28-31). In a retrospective multicenter study Vandersteen et al. (28) outlined only 5/745 (0.7%) cases of urethral obstruction. Christen et al. (32) reported a case of VUR endoscopically treated with Dx/HA with the longest interval between injection and the appearance of obstruction. Ben-Meir et al. (33) believed that in high-grade reflux as well as dilated ureter cases, because of the higher risk of late obstruction, surgery is indicated so endoscopy cannot be determined as proper therapeutic choice. Zyczkowski et al. (14) reported two cases of persisted reflux and UTI resistant to antibiotics with the histopathology of long-lasting inflammation, granuloma, and pseudocysts around the injection site of Dx/HA. Another investigation demonstrated that Dx/HA injection causes a granulomatous inflammation reaction with the existence of CD86 and tryptase positive cells with an abnormal stock of collagenous stroma and remodeling of ECM through myofibroblast differentiation, myofibroblasts cause tissue contraction with urethral diameter reduction and modifying the ratio of urethral length to diameter. This will result in urine reflux prevention (34).
Dexell® is another agent that is composed of hyaluronic acid gel (17 mg versus 15 mg in Deflux®) but with different size of dextranomer microspheres (80 - 120 µm and 80 - 250 µm in Deflux®) (27). A study compared these two similar agents, and reported that the success rate was 75.2% for Deflux® versus 71.8% in Dexell® that were not statistically different (P > 0.05). The mean age of treated children was 78 months, and they concluded that younger age and previous history of failure in injection is associated with unfavorable results. There was no difference in outcomes and UTI for these two agents (27).
2.2. Polydimethylsiloxane
Polydimethylsiloxane as a non-biodegradable material was first used in 1994 to treat children with VUR. Histopathological experimental investigations showed the development of a well-encapsulated foreign body reaction at the injection site, including giant cells, fibroblasts, and collagen, with no risk of migration because of the size of the particles (100 - 200 µm in diameter) (8, 35, 36). Distant migration is the consequence of phagocytosis by macrophages or circulating monocytes. Because macrophages do not phagocyte particles greater than 80 µm in diameter, PDS is safe from phagocytosis (35). Moreover, compared with other agents, the higher viscosity and the absence of shrinkage make PDS more reliable (37). The success rate of VUR endoscopic treatment with PDS is reported form 51.6% by Mohamed et al. (38) to 90% by Herz et al. (39). The low success rate was considered due to inexperienced operative’s inaccuracies in selecting the injection site or depth and preoperative bladder detrusor instability and voiding dysfunction that should be treated before endoscopic injection. The UTI incidence in their follow-up was also high (60%) which might be related to sex like 70% of their patients were females. Other reports represented the success rate between 70% and 90% and UTI incidence of < 20% (40-43). A case of symptomatic obstruction following PDS injections was reported which was attributed to an excess amount of material injected (40). There was also one technical error of nearly complete loss of PDS freely floating on the bladder floor in the 3-month follow-up. The reason was suggested to be the too superficial injection or excessive fluidity of substance (41). The most common complication with Macroplastique® injection was the occurrence of contralateral denovo reflux in the unilateral cases in Herz et al. (39). They reported de novo contralateral grades I and II reflux developed in 3% of children (17).
2.3. Polyacrylate-Polyalcohol Copolymer
Polyacrylate-polyalcohol copolymer (PPC) that belongs to the acrylic family results in the formation of the fibrotic capsule with good stability and long-term durability in the endoscopic treatment of VUR. PPC particles are flexible with the irregular shape when compressed. Most of the particles have an average of 300 µm diameter with low risk of migration (44). There are few studies with PPC as the bulking agent in VUR. The mean volume of the injected agent was 0.6-1 mL in different studies. The cure rate was reported as 77% to 94.9% (45-49). Postoperative UTI incidence was < 10% in most studies, but Chertin et al. (46) reported a 13% incidence of UTI after endoscopic injection of PPC. These studies showed that PPC has a high level of efficacy and safety in grades I-IV VUR. Chertin et al. (47) reported urethral obstruction in two patients, treated with stent insertion in one case and open urethral implantation in another. Ormaechea et al. (49) encountered a case of progressive hydronephrosis on ultrasonography after a urinary infection that was resolved by extravesical ureteral reimplantation.
2.4. Poly (Lactic-co-Glycolic Acid)
Poly (lactic-co-glycolic acid) (PLGA) due to its biodegradability and biocompatibility can be used in the different therapeutic applications such as tissue engineering scaffolds (50), cell culture substrate (51), and drug delivery vehicle (52, 53). This copolymer is synthesized by co-polymerization of cyclic dimers (1,4-dioxane-2,5-diones) of glycolic acid and lactic acid (54). Cho et al. (55) have injected PLGA in the submucosal layer of 6 rabbit bladders. They encountered no needle obstruction. Scanning electron microscope (SEM) images showed spherical structures with a slick surface. Gross views and histological analyses showed the formation of a hybrid tissue. Hematoxylin and eosin (H & A) staining showed host cells from the surrounding bladder muscle tissue migrated and filled the space between the microspheres. Masson’s trichrome staining indicated hybrid tissue containing collagen. They suggested that PLGA microspheres could be a potentially beneficial bulking agent for urologic injection therapies. The potential role of PLGA microspheres in combination with hyaluronic acid also can be considered as a urologic bulking agent, but no clinical trials regarding these agents were found (56).
2.5. Polyacrylamide Hydrogel
Polyacrylamide hydrogel is a lucid gel with 97.5% apyrogenic water and 2.5% cross-linked polyacrylamide. PAHG can be used as a biocompatible, non-resorbable, microparticle-free, and migration resistant bulking agent. Previous studies have shown the tissue integration, efficacy, and safety of PAHG (57-59). Ramsay et al. (60) have reported the PAHG cure rate of 70.7% for grade I-V VUR in a prospective study and a 12% incidence of UTI after injection. They did not exclude patients with renal duplication or previous history of endoscopic injections or ureteral reimplantation from the study that might have affected the final outcomes. Cloutier et al. (61) experienced a cure rate of 81.2% with up to 2 injections of PAHG. Only 2.5% of UTI was reported. No de novo or worsening hydronephrosis and no bulking agent calcification occurred in these two studies. PAHG has similar short-term efficacy to other bulking agents for the endoscopic resolution of VUR. PAHG costs less than other bulking agents such as Dx/HA so that it can be preferred over other bulking agents.
2.6. Collagen
Collagen injected endoscopically to treat VUR has a resolution rate of 93.9%, 91.7%, 85.3%, and 81.8% in one month, six months, two years, and four years, respectively (62). Studies revealed that in simple ureters, especially with grade III VUR, collagen can treat the reflux. Haferkamp et al. (63) reported a 95% success rate on the first postoperative voiding cystourethro-gram (VCUG). However, after 37 months, only 9% of their patients were reflux free. So, they concluded that a single endoscopic bovine collagen is not effective in the long-term for the treatment of VUR and the recurrence rate is high (63).
2.7. Polytetrafluoroethylene
Polytetrafluoroethylene is a chemical substance formed by tetrafluoroethylene gas polymerization at high temperature and pressure. Ninety percent of the particles have a diameter smaller than 40 µm, ranging from 4 - 100 µm (11). Aragona et al. (64) believed that the major concern is the migration of particles to regional lymph nodes and even other organs and caution is needed in the selection of patients for endoscopic VUR treatment with Polytetrafluoroethylene. Chertin et al. (65) declared a 58% and 84% resolution of refluxing units after first and second Polytetrafluoroethylene injection, respectively. One of their female patients encountered a unilateral ureteral obstruction and UTI incidence was 2.3%. In more than 18 years of follow-up, no unexpected clinical effects occurred (65).
2.8. Polymethylmethacrylate/Dextranomer
The polymethylmethacrylate/dextranomer (PMMA/Dx) agent comprises 15% PMMA and 75% cross-linked dextran. Korean FDA in 2010 have approved its application for soft tissue augmentation. Cross-linked dextran and PMMA microspheres have diameters of 45 to 120 mm and 32 to 120 mm, respectively. They cannot be phagocytized due to the large molecular size. PMMA/Dx as a bulking agent is biocompatible, nonresorbable, and migration free (66). PMMA/Dx revealed better shape and volume maintenance after three weeks compared to Dx/HA in an animal study, and inflammatory cells or macrophages’ infiltration did not happen at the injection site with PMMA/Dx (67). Kim et al. (66) displayed well-positioned PMMA/Dx material at the injection site and no obstruction on postoperative ultrasound during a short-term follow-up period (3 months) of 18 children. They reached a 69.7% of success rate with PMMA/Dx, which is in the range of Dx/HA injection outcomes. They reported urinary retention in one patient improved after two weeks of urinary catheterization and mild pyelectasis in another patient which spontaneously resolved after three months.
2.9. Calcium Hydroxyapatite
Hydroxyapatite (HA) as mineral structure of bones and teeth has a broad range of applications in orthopedics and dentistry fields. Coaptite which is mostly consisted of HA has been approved by the FDA for the treatment of stress urinary incontinence in women (68-70). Because of the radiopacity feature of calcium hydroxyapatite (CaHA), it is easy to assess the migration of this bulking agent. Mevorach et al. (71) conducted the first study of CaHA in the treatment of VUR. The third months’ success rate for grades II, III, and IV VUR was 95, 55, and 42%, respectively, and no significant side effect was reported. Only one case with transient obstruction was reported which resolved spontaneously within 48 hours. Tanhaeivash et al. (72) applied CaHA in VUR patients with febrile UTI and success rates of 52.5, 70, and 87.5% were achieved after the first, second, and third injection, respectively. Febrile UTI was resolved in 85% of cases. Other studies reported cure rates of 40% - 90% following CA injection (73-75). One serious complication following CaHA migration was high-grade hydronephrosis with loss of parenchyma on the left kidney resulting in nephroureterectomy because of a non-functioning kidney (75).
2.10. Autologous Injectable Agents
Mammalian autologous chondrocytes injection as a bulking agent for endoscopic therapy of VUR was reportedly successful to some extent (76). Autologous chondrocytes can form viable cartilage and form a stable submucosal bulk of tissue (77). Three-months and one-year success rate of chondrocyte suspensions harvested from articular cartilage injected as a bulking agent were 79% (78), and 70% (79), respectively. It is shown that myoblast transplantation can also regenerate damaged and degenerated tissue. The efficiency of transurethral injections of small depots of autologous myoblasts and fibroblasts in the treatment of urinary incontinence were observed (78). However, Mitterberger et al. (80) reported that in a vast bulk of cells, most cells become necrotic and myoblasts cannot be used as a bulking agent.
2.11. Tissue-Engineered Bulking Agents
Tissue engineering with the concept of cell transplantation, material science, and biomedical engineering aims to build biologic substances for the substitution of damaged or defunct tissues and organs with normal or near-normal function. To induce regeneration, functional cells are injected into the nonfunctional area. Scaffolds are synthetic or natural matrices, which stimulate the natural ability of the body to self-repair and guides the appropriate orientation and direction of tissue growth (81).
Tissue-engineered bulking agents have been used in the genitourinary tract for the formation of new, functional urologic structures (82). It uses selective autologous cell transplantation to create new functional, non immunogenic tissue that can survive in vivo. Before implantation, donor tissue is harvested and dissociated into individual cells or small tissue segments. This technique expands cultured cells to great numbers before their reimplantation. So, their use in surgical reconstruction is possible and the surgeon can rebuild almost normal anatomy in a physiologically functional tissue. A living, injectable, tissue-engineered autologous bulking agent can be used in the perseverance of vesico-ureteral reflux. Kershen et al. (3) showed promising experimental results obtained with tissue-engineered autologous chondrocytes and muscle cells.
2.12. A New Tissue-Engineered Bulking Agent
Today, the natural extracellular matrix (ECM) is widely used in cellular and tissue engineering in various forms (15-17). Various types of materials or tissue structures based on ECM have been fabricated for regeneration purposes. According to recent studies (83), a significant reduction of complications has attracted attention to using these substances. In this regard, we have been looking for a natural scaffold for infants and children, with fewer complications. Out of various tissues that are processed for this purpose, we chose prepuce tissue. Prepuce tissue can be stored in the tissue bank, after birth and circumcision, under sterile conditions and it could undergo the process of acellularization, lyophilization and micronization to be injected, in the related pathologic condition.
2.13. Decellularization and Recellularization Process
We obtained the prepuce from circumcising normal boys in the pediatric urology operating room. First, samples were washed with phosphate-buffered saline (PBS) for 30 minutes and were placed in sodium dodecyl sulfate (SDS) 2% solution for 20 minutes, followed by 1% triton X-100 solution for one hour. In the next phase, the tissues were immersed in a trypsin ethylene diamine tetraacetic acid (EDTA) solution at 37°C for 10 minutes. To ensure the efficacy of decellularization process, samples were then stained with hemotoxylin and eosin (H & E) for general observation, Masson’s trichrome for ECM architecture evaluation, and DAPI (4’,6-diamidino-2-phenylindole) to ensure the nuclear residue removal.
To evaluate cell adhesion and cytotoxicity, the recellularization of decellularized tissue was done with adipose-derived mesenchymal stem cells (ASCs). We isolated ASCs, as described in our previous study (84). Decellularized tissues were prepared (1 cm × 1 cm) and were sterilized with PBS buffer containing antibiotics. After washing with PBS for three times, the decellularized prepuce tissues were seeded with ASCs at the density of 1 × 104 and were incubated at 37°C, 5% CO2 for 3 hours to allow cell adhesion on the surface of decellularized tissues. Afterward 1 mL of 10% Dulbecco’s modified Eagle medium/fetal bovine serum (DMEM/FBS medium) was added to the specimens and was kept in an incubator. After 48 hours of incubation, the specimens were transferred to new plates and were stored in an incubator for two weeks. The culture medium was substituted every two days. Then, the samples were washed with PBS and immersed in 10% NBF for histopathological evaluations. We selected rabbits for this experimental study.
2.14. In Vivo Study
For in vivo study, eight rabbits were randomly divided into two groups. In the first group 0.2 cc of lyophilized and micronized prepuce and in the second group 0.2 cc of Urodex® (viscous gel consisting of a suspension of dextranomer-microparticles and cross-linked hyaluronic acid) was injected into the seromuscular wall of the bladder. Microscopic examination of acellular prepuce compared with normal tissue showed the success of this process with no evidence of cellular remnants in the acellular tissue. Immunohistochemistry staining with CD68 and LCA revealed a higher inflammation grade in Urodex® as compared with Prepuce. The results of this study showed that lyophilized and micronized prepuce could be an operative alternative to Urodex® as a natural and non-synthetic bulking agent in the treatment of children with vesicourethral reflux.
Prepuce tissue is biocompatible and durable. This tissue also is more structurally similar to the urinary tract system (85). Due to the importance of the application of this material in infants and children, the human phase was postponed to evaluation and achieving favorable results by histopathologic and immunologic evidence of animal phase.
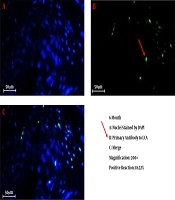
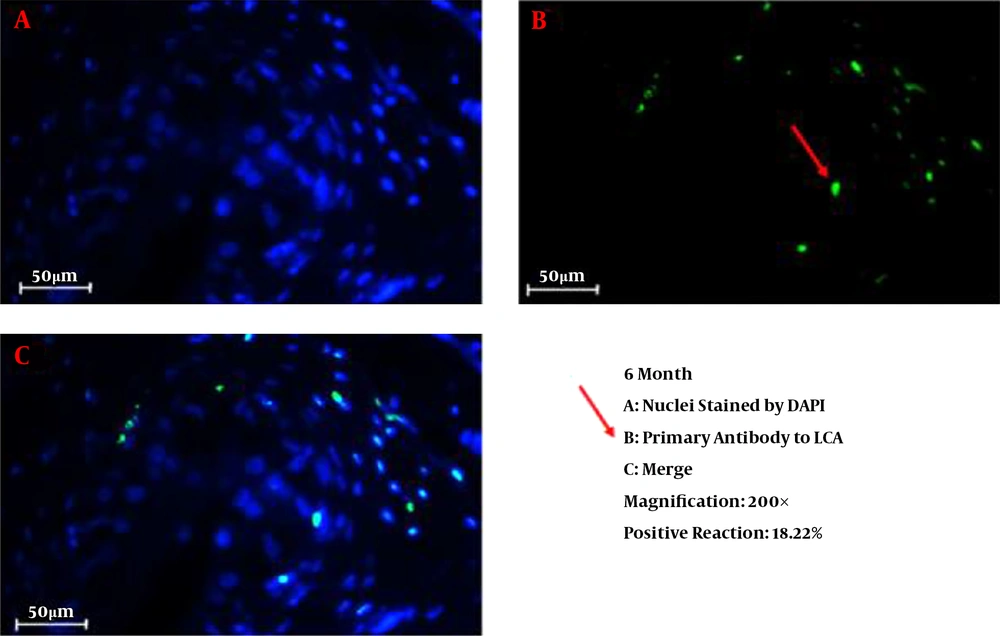
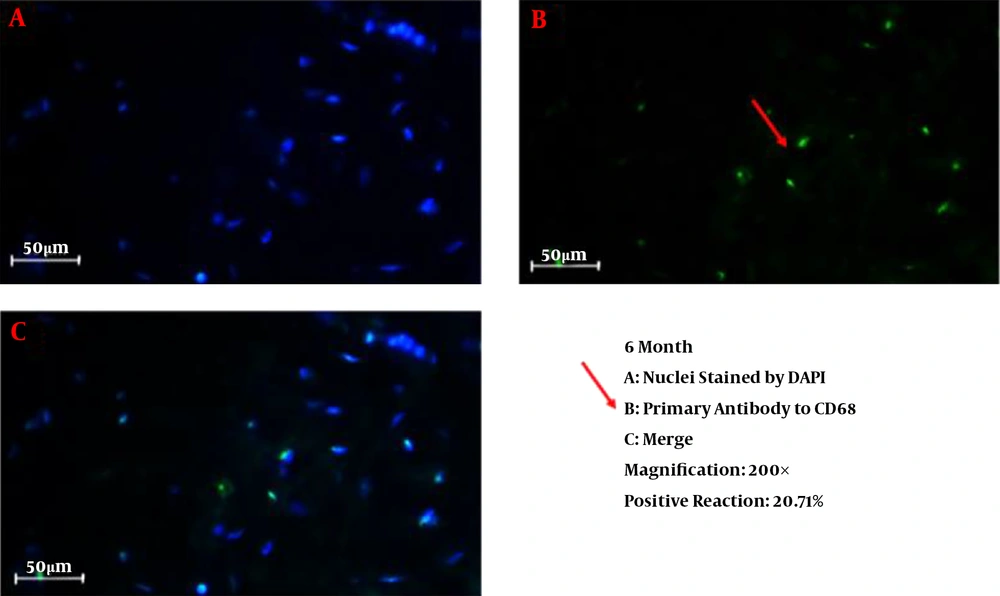
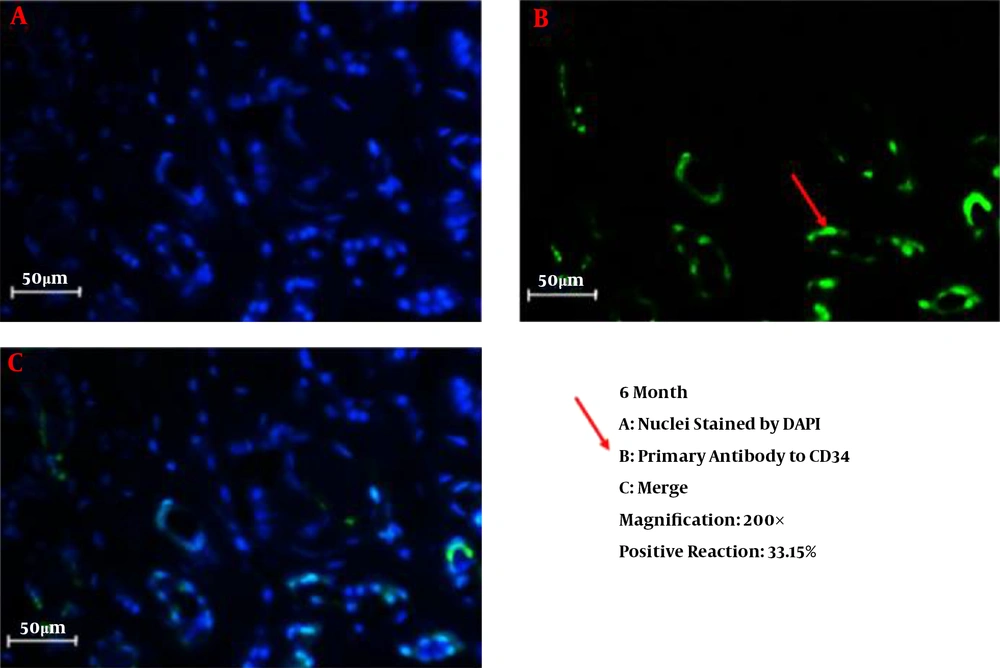
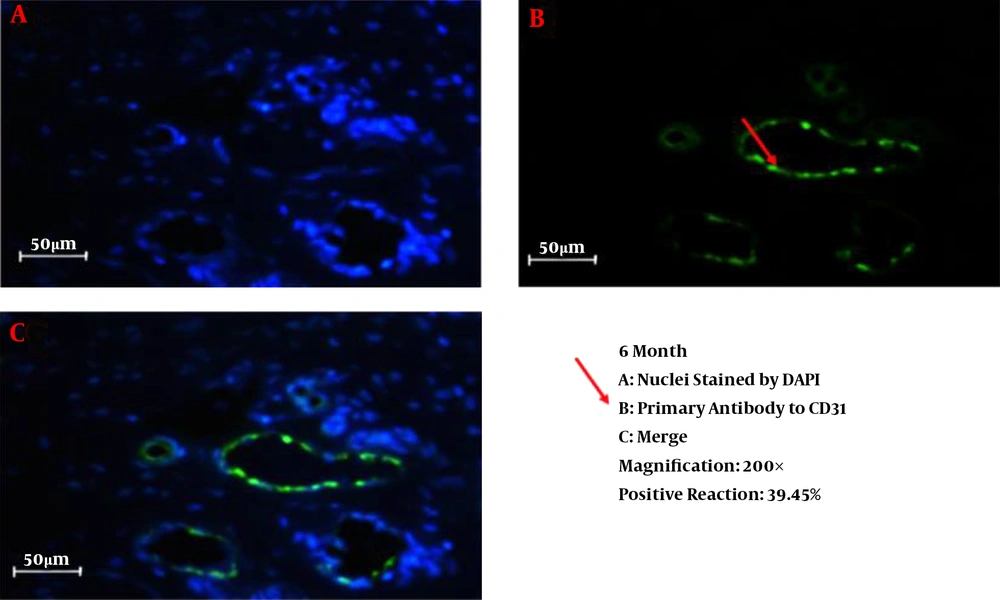
We successfully conducted the process of decellularization of prepuce tissue. This newly engineered bulking agent could have different advantages such as easy process and culture, excellent biocompatibility and degradability, and rarely induces immunoreaction and inflammatory responses. Long-term sustainability after endoscopic injection makes this tissue-engineered bulking agent a potential treatment for VUR. Also, our previous study showed that the submucosal injection of tissue-engineered micronized and lyophilized prepuce tissue is easily performed, well-tolerated, safe, and effective with appropriate long-term durability. We investigated the development of fibrosis and inflammation of the tissues by inflammatory markers such as leukocyte common antigen (LCA), CD31, CD34, and CD68. The results confirmed that the inflammation rate and the incidence of fibrosis in the injected site of the prepuce tissue in the bladder were similar to Deflux®. In addition, the CD31 and CD34 staining, which illustrate endothelial regeneration and angiogenesis development (86), did not show significant differences between our new bulking agent and Deflux® one month after injection. Immunofluorescence staining disclosed that the inflammation and fibrosis rate decreased by six months after injection in prepuce (leukocyte common antigen (LCA), CD31, CD34, and CD68 markers) (Figures 1-4). So the characteristics of tissue-engineered micronized and lyophilized prepuce tissue are more similar to Deflux® as a gold standard bulking agent.
Even though Deflux® is a good candidate for bulking agents, since it is synthetic with chemical ingredient it is more considerable to find an agent with close characteristics to the body structure. Therefore, tissue-engineered lyophilized prepuce can be considered as an appropriate alternative to Deflux®. It has similar characteristics to Deflux® and lower prices, in addition, it can also be used in both genders due to being decellularized.
3. Conclusions
Various foreign materials have been used for endoscopic suburethral injections, and their success rate has been different from 40 to > 90 percent. However, there are documented disadvantages related to these materials in some studies such as granuloma formation (10, 11), migration to distant organs (12), autoimmune reactions, and early recurrence of VUR (9). Dx/HA, currently the most common bulking agent used for VUR resolution, is an artificial material that helps to grow collagen and fibroblasts. Activation of the body’s defense system and the formation of granuloma pseudocysts around the Dx/HA are complications after the injection of this agent. Therefore, despite the better outcomes in comparison to other agents, the use of this material might be associated with the risk of appearance of serious and persistent complications (14). Another problem with Dx/HA in our country is its high cost. In order to find an alternative bulking agent, we formed decellularized prepuce tissue as a biocompatible tissue-engineered bulking agent and showed that it is comparable to Dx/HA regarding low immunoreaction and inflammatory responses with lower price. Although, it could be the criterion of an ideal bulking agent for the future clinical application, it is necessary to perform further studies on the histopathological changes associated with this bulking agent and also long-term follow-ups in children to assess potential complications.